Abstract
Objective
To characterize hyperosmolarity-responsive genes in leiomyoma cells and determine whether gonadotropin-releasing hormone (GnRH) agonist treatment altered their expression.
Design
Laboratory study.
Setting
University hospital.
Patient(s)
None.
Intervention(s)
Cell culture under hypertonic conditions and with GnRH agonist treatment, RNA isolation, and real-time reverse-transcriptase polymerase chain reaction (RT-PCR).
Main Outcome Measure(s)
Expression of nuclear factor of activated T cells 5 (NFAT5), aldose reductase (AR), and sodium myo-inositol transporter 1 (SMIT) messenger RNA (mRNA) in immortalized leiomyoma and patient-matched myometrial cells.
Result(s)
Leiomyoma cells had increased basal expression of NFAT5 mRNA (1.7 ± 0.08-fold) compared with myometrial cells. The NFAT5 increased further in leiomyoma cells cultured under hyperosmolar conditions (3.0 ± 0.46-fold at 50 mM NaCl and 3.3 ± 0.48-fold at 100 mM NaCl). The NFAT5-regulated mRNA transcripts for AR and SMIT were increased in untreated leiomyoma cells compared with myometrial cells and further increased in leiomyoma cells exposed to osmotic stress. The NFAT5 transcripts were decreased with low-dose GnRH agonist treatment but increased with supraphysiologic doses.
Conclusion(s)
Expression of hyperosmolarity genes was increased in leiomyoma cells relative to myometrial cells. Pharmacologic concentrations of GnRH agonist decreased NFAT5 expression, suggesting that water flows out of leiomyoma cells at pharmacologic doses.
Keywords: Leiomyoma, leuprolide acetate, myometrium, NFAT5, osmotic stress
Uterine leiomyomas are the most common gynecologic tumors in reproductive-aged women and are the leading indication for hysterectomy in the United States (1–3). These benign tumors cause dramatic morbidity including pelvic pain, bleeding, infertility, miscarriage, and preterm labor (4–9). The economic impact of leiomyomas is great, with the total direct cost to the U.S. health care system estimated at $2.1 billion per year (10). The only medical therapy approved by the U.S. Food and Drug Administration (FDA) for uterine leiomyomas is the gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate (11). After 3 to 6 months of treatment with a GnRH agonist, uterine volume is reduced 25% to 50% (12, 13). Though significant leiomyoma reduction occurs after treatment with a GnRH agonist, rapid regrowth usually occurs within 6 months of stopping treatment (14, 15). The clinical effects of GnRH agonist treatment are well-known, but the molecular mechanisms of GnRH action on leiomyomas are incompletely understood.
Leiomyomas are characterized by excessive and disorganized extracellular matrix (ECM) production (16). Excess ECM deposition and fibrosis are the key features of leiomyomas, which contribute to the stiffness observed in these tumors (17, 18). Hydrophilic proteoglycans are a major component of the ECM and may also contribute to the increased stiffness of leiomyomas (19). Altered expression of ECM genes, proteases, protease inhibitors, and growth factors in GnRH-treated leiomyomas suggests that ECM degradation or remodeling is one mechanism of GnRH-analogue action (20–22). Relatively rapid reduction of leiomyoma volume and rapid regrowth with discontinuation of treatment suggest that mechanisms other than ECM remodeling may be involved in these GnRH-mediated effects. Furthermore, leiomyomas are characterized by low mitotic activity (23), thus reduction in cell proliferation is an unlikely explanation for the GnRH-mediated reduction in leiomyomas. Leiomyoma cells express GnRH receptors (24, 25), suggesting that GnRH analogues may regulate leiomyomas through a direct interaction. One possible explanation is that GnRH treatment causes water efflux from leiomyoma cells, resulting in a reduction in leiomyoma volume.
Maintenance of water homeostasis is critical to the survival and function of mammalian cells. Under conditions of extracellular hyperosmolarity (osmotic stress), cells lose water and consequently shrink, leading to a crowding of cellular contents and impairment of intracellular functions. The transcription factor, nuclear factor of activated T cells-5 (NFAT5), is a key regulator of the osmotic stress response in mammalian cells (26–28). In response to hyperosmotic stress, NFAT5 maintains water homeostasis by increasing the expression of hyperosmolarity-responsive genes such as aldose reductase (AR) and sodium myo-inositol transporter 1 (SMIT), which increases intracellular osmolarity and leads to up-take of water into cells. Aldose reductase catalyzes the reduction of glucose to sorbitol, and SMIT transports inositol into cells (29, 30).
We hypothesized that GnRH-agonist treatment causes rapid changes in leiomyoma size due to a reduction in hyperosmolarity-responsive gene expression, leading to a loss of intracellular water. To test this hypothesis, we characterized osmoregulation in leiomyoma and myometrial cells and evaluated the cellular response to GnRH-agonist treatment.
MATERIALS AND METHODS
Cell Line
Approval was obtained from the institutional review board to collect tissue samples from patients undergoing medically indicated hysterectomy. Leiomyoma and myometrial tissues were collected at the time of surgery. Methods for tissue collection and generation of primary cultures were similar to those previously described elsewhere (31). Briefly, tissues were minced and digested with collagenase-2. The cells were centrifuged and resuspended in complete media containing Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS), 1X antibiotic, and 0.25 μg/mL Fungizone (GIBCO/Invitrogen, Grand Island, NY). Viable cells were plated in T-25 flasks, and fresh media was added to the flask every fourth day. Once the cells reached 50% confluence, the cells were trypsinized and plated for a second passage. When the cells reached 70% to 80% confluence, they were trypsinized and cryopreserved. Aliquots of the primary cultures of leiomyoma and myometrial cells were immortalized by transfection with recombinant retrovirus containing the E6/E7 genes of human papillomavirus (HPV) type 16 (32).
Osmotic Stress Experiments
Immortalized leiomyoma and myometrial cells were cultured in complete media composed of Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) (Invitrogen) with 10% fetal bovine serum (FBS), penicillin-streptomycin-neomycin (PSN), and amphotericin B at 37°C in the presence of 5% CO2. Based on published reports, we selected NaCl to induce osmotic stress (26, 27, 33, 34). Hypertonic media was prepared by adding NaCl from a 5 M stock solution to complete media to make concentrations of 25, 50, 75, and 100 mM NaCl. First, the cells were subcultured in isotonic media in 48-well plates at 2.5 × 103 cells/well. Once the cells reached 30% confluence, the isotonic media was removed, and media containing additional osmolytes of NaCl was added to the plated cells. Two plates for each cell line were collected after 24, 48, 72, and 96 hours of exposure to the hyperosmotic conditions. Cell proliferation was determined using the sulforhodamine-B method (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions. Proliferation studies were performed in triplicate with six replicates of each concentration per plate.
Additional leiomyoma and myometrial cells were added to six-well plates at 2 × 104 cells/well. Once the cells reached 80% confluence, the isotonic media was replaced with hypertonic media containing an additional 50 mM or 100 mM NaCl. Cells were collected after 8 and 24 hours of culture in the hypertonic media and stored for RNA isolation.
GnRH Treatment Experiments
Leiomyoma and myometrial cells were cultured in complete media at 37°C in the presence of 5% CO2. Cells were added to six-well plates at 2 × 104 cells/well. Cells were grown for 48 hours in phenol red-free, complete media with 10% FBS, then quiescence was induced by incubating the cells in phenol red-free, serum-free media for 48 hours. The GnRH agonist, leuprolide acetate (Sigma-Aldrich), was added to phenol red-free media containing 10% charcoal-treated FBS to make 1 and 10 μM solutions. These experiments were based on prior investigations demonstrating resumption of proliferation and gene expression in serum-starved leiomyoma cells when serum containing media was restored (35, 36). Cells were treated with leuprolide and then collected after 24 hours of treatment and stored for RNA isolation.
RNA Isolation
The RNA from the osmotically stressed and GnRH-agonist–treated cells was isolated by lysis of adherent cells with TriZol (Invitrogen), followed by chloroform extraction (0.2 mL/mL of TriZol). The samples were centrifuged at 12,000 × g for 15 minutes at 4°C. To precipitate the RNA, ice-cold isopropanol was added at 0.5 mL per mL TriZol. The RNA pellet was washed once with 70% ethanol, dried, and suspended in water treated with diethylpyrocarbonate (DEPC). To remove any contaminating DNA, all RNA samples were treated with DNAse-I enzyme using the DNA-free kit (Ambion, Austin, TX), in accordance with the manufacturer's instructions. The RNA integrity and concentration were confirmed by electrophoresis and spectrophotometry readings measured at A260 and A280. The RNA samples were stored at −80°C.
Real-Time RT-PCR
Total RNA isolated from treated and untreated leiomyoma and myometrial cells was diluted into 100 ng/μL and 10 ng/μL stock concentrations. Primers and probes for NFAT5, AR, and SMIT were designed using Beacon Designer (Premier Biosoft, Palo Alto, CA) to amplify a 90 to 150 base pair (bp) section near to the 3' end of the translated region (within 500 bp). The iScript One-Step RT-PCR kit (BioRad, Hercules, CA) was used, and thermal cycling was performed with the BioRad iCycler iQ Real-Time PCR Detection System (BioRad). The 18s ribosomal RNA was used as an internal standard to scale the measurements of the different samples. All experiments were performed in triplicate. Data were analyzed with iCycler iQ software (BioRad).
Statistical Analysis
A Wilcoxon signed rank sum test was used for analysis of significance of the gene and protein expression. Data were calculated as a fold difference in expression compared with controls and are presented as mean ± standard error of the mean. P<.05 was considered statistically significant.
RESULTS
Osmoregulation mediated by NFAT5 has been described in multiple cell types (37–40) but not in leiomyoma cells. Before evaluating the osmotic response, we first characterized the basal expression of the hyperosmolarity-responsive genes in leiomyoma and myometrial cells. To quantify NFAT5 and NFAT5-activated gene expression, real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed for NFAT5, AR, and SMIT transcripts, comparing leiomyoma with myometrial cells. The NFAT5 mRNA expression was 1.7-fold higher in untreated leiomyoma cells compared with untreated myometrial cells. The NFAT5-activated genes AR and SMIT were increased 2.2-fold and 2.3-fold, respectively, in untreated leiomyoma cells compared with untreated myometrial cells.
Leiomyoma cells cultured under hyperosmolar conditions had further increases in hyperosmolarity-responsive gene transcripts. Under osmotic stress at 50 mM of NaCl, NFAT5 transcripts were increased 3.5-fold in leiomyoma cells, compared with myometrial cells cultured without additional NaCl (P<0.05), and they remained increased under higher osmotic stress (100 mM NaCl) (Fig. 1). In myometrial cells, the NFAT5 transcripts increased 2.9-fold in 50 mM of NaCl, but with higher osmotic stress, the transcripts fell to the level of untreated leiomyoma cells (P<0.05). This suggests that leiomyoma cells produced a more robust response to osmotic stress through induction of the hyperosmolarity-responsive genes compared with myometrial cells.
FIGURE 1.
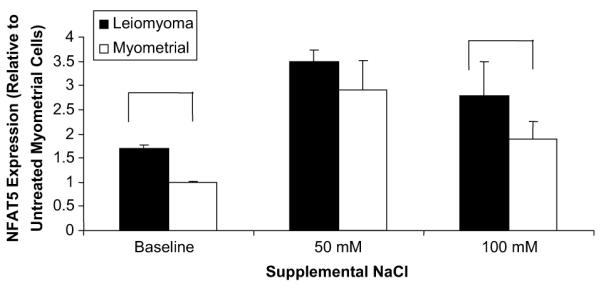
Nuclear factor of activated T cells 5 (NFAT5) expression in leiomyoma and myometrial cells. Leiomyoma cells had a higher expression of NFAT5 messenger RNA (mRNA) compared with myometrial cells. Data represent the mean relative expression of NFAT5 mRNA shown as the fold difference compared with myometrial cells at baseline. Error bars indicate the standard error of the mean. Data shown represent the average of three experiments.
McCarthy-Keith. Osmoregulation in leiomyoma cells. Fertil Steril 2011.
To determine whether the increased NFAT5 led to an increase in gene targets in leiomyoma cells, we tested the hyperosmotic response gene targets of NFAT5. Under osmotic stress, transcripts for AR mRNA were further increased: 5.7-fold at 50 mM and 5.4-fold at 100 mM of NaCl compared with untreated myometrial cells. At 50 mM NaCl, transcripts for SMIT were similar to baseline levels, but were increased 5.3-fold under higher osmotic stress (Fig. 2). These results indicate that, at 50 mM NaCl, leiomyoma cells induced a greater abundance of the NFAT5-targeted genes under hyperosmolar conditions.
FIGURE 2.
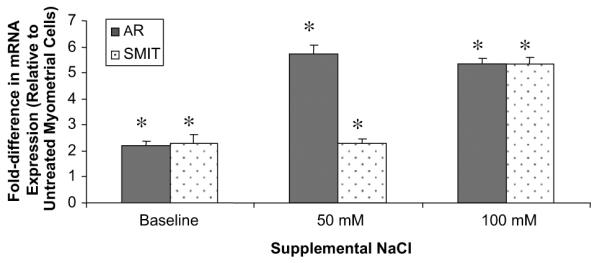
Hyperosmolarity-responsive gene expression in leiomyoma cells. Aldose reductase (AR) and sodium myo-inositol transporter 1 (SMIT) transcripts were increased at baseline compared with myometrial cells. Transcripts for AR increased at 50 and 100 mM NaCl, and SMIT transcripts increased only under higher osmotic stress conditions. Data shown are the mean ± standard error of the mean of the fold difference in mRNA expression in leiomyoma cells relative to myometrial cells at baseline and under hyperosmolar conditions. Values represent the average of three experiments. *P<.05 relative to myometrial cells at baseline.
McCarthy-Keith. Osmoregulation in leiomyoma cells. Fertil Steril 2011.
Next, we conducted proliferation studies of leiomyoma and myometrial cells cultured under hyperosmolar conditions to determine whether cell proliferation would be affected by osmotic stress (Fig. 3). As expected, the addition of osmolytes led to a dose-dependent reduction in proliferation of both leiomyoma and myometrial cells. However, with 50 mM of added NaCl, cell growth was inhibited 48% in myometrial cells versus 17% in leiomyoma cells, suggesting that leiomyoma cells were more resistant to hyperosmotic stress (P<.05).
FIGURE 3.
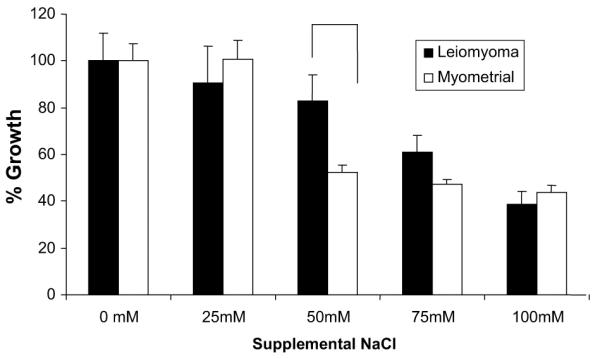
Leiomyoma and myometrial cell proliferation under osmotic stress. Cell growth was inhibited in a dose-dependent manner in both cell types. At 50 mM NaCl, leiomyoma cells demonstrated greater resistance to osmotic stress. Leiomyoma and myometrial cells were cultured in media with added NaCl (x axis). Leiomyoma and myometrial cell proliferation are depicted as the percentage growth (y axis) relative to cell proliferation under isotonic conditions (0 mM supplemental NaCl) in leiomyoma and myometrial cells, respectively. This figure depicts cell proliferation at 24 hours and is representative of proliferation curves at 48, 72, and 96 hours. Proliferation studies were performed in triplicate, and the representative data are shown. Data shown represent the average rate of growth ± standard error of the mean. Proliferation was determined by the sulforhodamine B assay method.
McCarthy-Keith. Osmoregulation in leiomyoma cells. Fertil Steril 2011.
The proliferation studies were repeated, with the leiomyoma and myometrial cells returned to isotonic culture media after osmotic stress. Growth was inhibited in both cell types under hypertonic conditions, but proliferation resumed when the hypertonic media was replaced with normosmotic media (data not shown). These findings suggest that osmotic stress produced a cytostatic effect in the leiomyoma and myometrial cells, rather than a cytotoxic effect. Based on these studies, which demonstrated a differential effect of NaCl on leiomyoma and myometrial cell growth beginning at 50 mM and a marked effect on both cell types at 100 mM, we selected the same concentrations to induce hypertonicity for subsequent experiments.
Having characterized the response to hyperosmolarity, we next evaluated the direct effect of GnRH-analogue treatment on the expression of the hyperosmolarity responsive genes in leiomyoma cells. In leiomyoma cells treated with pharmacologic concentrations of leuprolide (0.001 and 0.01 μM), we observed a reduction in NFAT5 transcripts as compared with untreated leiomyoma cells (Fig. 4). At supraphysiologic concentrations, NFAT5 expression was increased, which demonstrated a biphasic response of leiomyoma cells to leuprolide treatment. We confirmed the increased expression of the hyperosmolarity-responsive gene mRNAs in leiomyoma cells treated with 1 μM and 10 μM concentrations of leuprolide.
FIGURE 4.
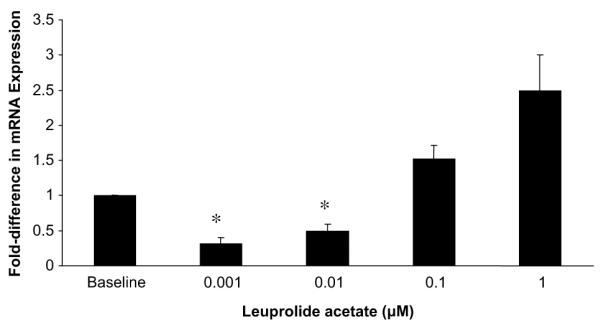
Nuclear factor of activated T cells 5 (NFAT5) messenger RNA (mRNA) expression in leiomyoma cells treated with leuprolide acetate. The NFAT5 transcripts were decreased at pharmacologic concentrations (0.001 and 0.01 μM). Leiomyoma cells were cultured in phenol-free, charcoal-stripped media, followed by incubation phenol red-free, serum-free media for 48 hours. Cells were then treated with varied concentrations of leuprolide (x axis) added to phenol red-free, charcoal-stripped media for 24 hours and then harvested. Data shown represent the average NFAT5 mRNA abundance in treated leiomyoma cells, presented as the fold difference in mRNA expression relative to leiomyoma cells at baseline (y axis). Error bars indicate standard error of the mean. *P<.05 relative to leiomyoma cells at baseline.
McCarthy-Keith. Osmoregulation in leiomyoma cells. Fertil Steril 2011.
DISCUSSION
We examined the osmotic response of leiomyoma and myometrial cells and found that, at isotonic conditions, leiomyoma cells expressed increased amounts of NFAT5 transcripts compared with my ometrial cells. Exposure of both cell types to osmotic stress further induced the expression of NFAT5, although NFAT5 mRNA was more abundant in leiomyoma cells at basal conditions and at states of increased extracellular hyperosmolarity. The greater abundance of NFAT5 in leiomyoma cells suggests that at baseline the leiomyoma cells are exposed to a hyperosmolar extracellular milieu. The elevation of the NFAT5 targets, AR and SMIT, supports that interpretation. Preliminary protein analysis suggested a greater abundance of NFAT5, AR, and SMIT proteins under hyperosmolar conditions.
It is possible that leiomyoma cells are under osmotic stress in vivo. Rogers et al. (17) observed that increased mechanical loading on leiomyoma cells was associated with increased levels of AKAP13. Kino et al. (41) evaluated the osmotic stress response in immune cells and demonstrated that AKAP13 was critical for the expression of NFAT5 in response to extracellular hyperosmolarity. Taken together, these two studies suggest that AKAP13 might alter osmotic stress response in leiomyoma cells. We speculate that activation of osmotic stress signaling and induction of hyperosmolarity-responsive genes in leiomyoma cells results in water exchange between leiomyoma cells and the surrounding ECM.
The ECM is rich in hydrophilic proteoglycans, so the exchange of water between leiomyoma cells and the ECM is complex. Water lost by leiomyoma cells is either replaced through activation of ion and osmolyte transporters in leiomyoma cells or extracted from the ECM. This water exchange may contribute to the observed stiffness of leiomyomas as well as their decreased size in response to pharmacologic treatments. Our experiments did not include measurement of leiomyoma cell volume in response to osmotic stress; however, cell shrinkage in response to extracellular hyperosmolarity has been demonstrated in investigations of other cell types (42, 43).
Our observation of decreased expression of osmosensing genes in response to pharmacologic concentrations of leuprolide acetate demonstrates a direct effect of GnRH-agonist treatment and suggests that water outflow may explain the reduction in leiomyomas with pharmacologic GnRH treatment. Here we evaluated the direct effects of GnRH-agonist treatment, based on evidence that leiomyoma cells express GnRH receptors (24, 25). It is possible that indirect effects of GnRH may augment the osmotic response in leiomyoma cells. Future studies will investigate the possible indirect effect of GnRH-agonist treatment, specifically the effect of gonadal hormone treatments on the osmotic response in leiomyoma cells.
Our findings suggest that leiomyoma reduction and regrowth occur through water movement and that GnRH-agonist treatment alters this response as its mechanism of action. We describe basal differences in hyperosmotic response genes in leiomyoma and myometrial cells, suggesting that leiomyomas exist in a milieu of perceived hyperosmolarity. This highlights the potential importance of osmotic stress in the regulation of leiomyomas and their response to clinical treatment.
Acknowledgments
Supported in part by the Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and a research grant by Ferring Pharmaceuticals.
Footnotes
D.M.M-K. has nothing to disclose. M.M. has nothing to disclose. J.B. has nothing to disclose. J.S. has nothing to disclose. W.H.C. has nothing to disclose.
REFERENCES
- 1.Day Baird D, Dunson D, Hill M, Cousins D, Schectman J. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 2.Selo-Ojeme D, Lawal O, Shah J, Mandal R, Pathak S, Selo-Ojeme U, et al. The incidence of uterine leiomyoma and other pelvic ultrasonographic findings in 2,034 consecutive women in a north London hospital. J Obstet Gynecol. 2008;28:421–3. doi: 10.1080/01443610802149863. [DOI] [PubMed] [Google Scholar]
- 3.Merrill R. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–31. [PubMed] [Google Scholar]
- 4.Pritts E, Parker W, Olive D. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91:1215–23. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Bajekal N, Li T. Fibroids, infertility and pregnancy wastage. Hum Reprod Update. 2000;6:614–20. doi: 10.1093/humupd/6.6.614. [DOI] [PubMed] [Google Scholar]
- 6.Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology and management. Fertil Steril. 1981;36:433–45. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 7.Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107:376–82. doi: 10.1097/01.AOG.0000196806.25897.7c. [DOI] [PubMed] [Google Scholar]
- 8.Wegienka G, Baird DD, Hertz-Picciotto I, Harlow SD, Steege JF, Hill MC, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431–7. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil Steril. 2003;80:1488–94. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- 10.Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195:955–64. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration [Accessed March 28, 2011];FDA Drug Safety Communication: Ongoing safety review of GnRH agonists and possible increased risk of diabetes and certain cardiovascular diseases. 2010 May 3; Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatients andProviders/ucm209842.htm.
- 12.Friedman AJ, Harrison-Atlas D, Barbieri RL, Benacerraf B, Gleason R, Schiff I. A randomized, placebo-controlled, double-blind study evaluating the efficacy of leuprolide acetate depot in the treatment of uterine leiomyomata. Fertil Steril. 1989;51:251–6. doi: 10.1016/s0015-0282(16)60486-7. [DOI] [PubMed] [Google Scholar]
- 13.Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo controlled, multicenter study. Leuprolide Study Group. Obstet Gynecol. 1991;77:720–5. [PubMed] [Google Scholar]
- 14.Matta WH, Shaw RW, Nye M. Long-term follow-up of patients with uterine fibroids after treatment with the LHRH agonist buserelin. BJOG. 1989;96:200–6. doi: 10.1111/j.1471-0528.1989.tb01663.x. [DOI] [PubMed] [Google Scholar]
- 15.West CP, Lumsden MA, Lawson S, Williamson J, Baird DT. Shrinkage of uterine fibroids during therapy with goserelin (Zoladex): a luteinizing hormone-releasing hormone agonist administered as a monthly subcutaneous depot. Fertil Steril. 1987;48:45–51. doi: 10.1016/s0015-0282(16)59288-7. [DOI] [PubMed] [Google Scholar]
- 16.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–17. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers R, Norian J, Malik M, Christman G, Abu-Asab M, Chen F, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker CL, Stewart EA. Uterine fibroid: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 19.Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, et al. Transforming growth factor β3 regulates the versican variants in the extra-cellular matrix-rick uterine leiomyomas. Reprod Sci. 2009;16:1153–64. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dou Q, Tarnuzzer RW, Williams RX, Schultz GS, Chegini N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyo mata: a mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol Hum Reprod. 1997;3:1005–14. doi: 10.1093/molehr/3.11.1005. [DOI] [PubMed] [Google Scholar]
- 21.Luo X, Ding L, Xu J, Williams RS, Chegini N. Leiomyoma and myometrial gene expression profiles and their responses to gonadotropin-releasing hormone analog therapy. Endocrinology. 2005;146:1074–96. doi: 10.1210/en.2004-1384. [DOI] [PubMed] [Google Scholar]
- 22.Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig. 2003;10:161–71. doi: 10.1016/s1071-5576(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 23.Dgani R, Piura B, Ben-Baruch G, Open M, Glezerman M, Nass D, et al. Clinical-pathological study of uterine leiomyomas with high mitotic activity. Acta Obstet Gynecol Scand. 1998;77:74–7. doi: 10.1034/j.1600-0412.1998.770116.x. [DOI] [PubMed] [Google Scholar]
- 24.Parker JD, Malik MM, Catherino WH. Human myometrium and leiomyomas express gonadotropin-releasing hormone 2 and gonadotropin-releasing hormone 2 receptor. Fertil Steril. 2007;88:39–46. doi: 10.1016/j.fertnstert.2006.11.098. [DOI] [PubMed] [Google Scholar]
- 25.Chegini N, Rong H, Dou Q, Kipersztok S, Williams RS. Gonadotropin-releasing hormone (GnRH) and GnRH receptor gene expression in human myometrium and leiomyomata and the direct action of GnRH analogs on myometrial smooth muscle cells and interaction with ovarian steroids in vitro. J Clin Endocrinol Metab. 1996;81:3215–21. doi: 10.1210/jcem.81.9.8784072. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA. 2004;101:2392–7. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferraris JD, Williams CK, Persaud P, Zhang Z, Chen Y, Burg MB. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc Natl Acad Sci USA. 2002;99:739–44. doi: 10.1073/pnas.241637298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–74. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 29.Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–55. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiol Rev. 1991;71:1081–115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 31.Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87:1166–72. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- 32.Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol. 2008;69:462–70. doi: 10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drews-Elger K, Ortells MC, Rao A, Lopez-Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS One. 2009;4:e5245. doi: 10.1371/journal.pone.0005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Kim M, Im YS, Choi W, Byeon SH, Lee HK. NFAT5 induction and its role in hyperosmolar stressed human limbal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:1827–35. doi: 10.1167/iovs.07-1142. [DOI] [PubMed] [Google Scholar]
- 35.Chegini N, Ma C, Tang XM, Williams RS. Effects of GnRHanalogues, “add-back” steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle growth and transforming growth factor-β expression. Mol Hum Reprod. 2002;8:1071–8. doi: 10.1093/molehr/8.12.1071. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Luo X, Chegini A. Differential expression, regulation, and induction of Smads, transforming growth factor-beta signal transduction pathway in leiomyoma, and myometrial smooth muscle cells and alteration in gonadotropin-releasing hormone analog. J Clin Endocrinol Metab. 2003;88:1350–61. doi: 10.1210/jc.2002-021325. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q, Ferraris JD, Burg MB. High NaCl increases TonEBP/OREBP mRNA and protein by stabilizing its mRNA. Am J Physiol Renal Physiol. 2005;289:F803–7. doi: 10.1152/ajprenal.00448.2004. [DOI] [PubMed] [Google Scholar]
- 38.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol. 2002;22:5753–60. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loyher ML, Mutin M, Woo SK, Kwon HM, Tappaz ML. Transcription factor tonicity-responsive enhancer-binding protein (TonEBP) which transactivates osmoprotective genes is expressed and upregulated following acute systemic hypertonicity in neurons in brain. Neuroscience. 2004;124:89–104. doi: 10.1016/j.neuroscience.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Arroyo JA, Teng C, Battaglia FC, Galan HL. Determination of the NFAT5/TonEBP transcription factor in the human and ovine placenta. Syst Biol Reprod Med. 2009;55:164–70. doi: 10.3109/19396360902846401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kino T, Takatori H, Manoli I, Wang Y, Tiulpakov A, Blackman MR, et al. Brx mediates the response of lymphocytes to osmotic stress through activation of NFAT5. Sci Signal. 2009;2:ra5.1–15. doi: 10.1126/scisignal.2000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82:720–7. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–80. [PubMed] [Google Scholar]


