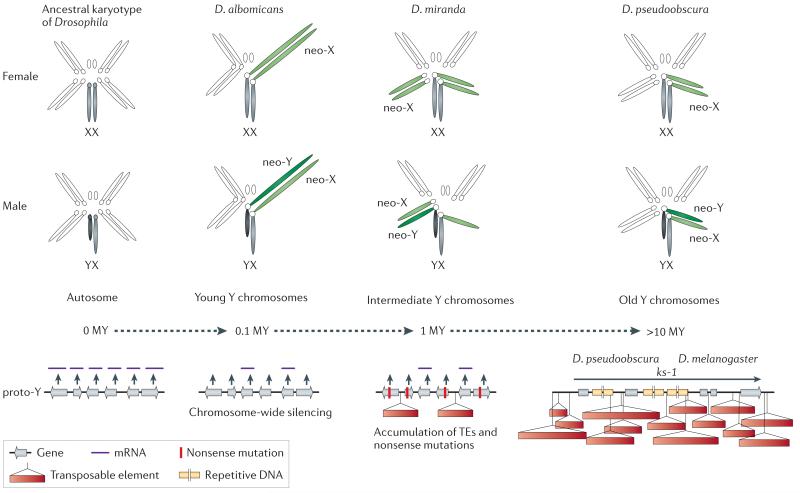
Figure 2. Neo-sex chromosomes in Drosophila.
Neo-sex chromosomes (in green) are formed by fusions of autosomes with the ancestral sex chromosomes (in grey), and the neo-X and neo-Y carry identical gene sets at the time of their origination (0 MY). To date, three Drosophila species with neo-Y’s of different age have been studied in detail, and provide a dynamic picture of the molecular changes associated with Y differentiation, and their karyotype is shown. Chromosome-wide down-regulation of protein-coding genes before accumulation of nonsense mutations at coding sequences is observed on the neo-Y in D. albomicans, which originated about 0.1 MY ago. Thus, gene decay on the neo-Y appears to be initiated by chromosome-wide silencing, possibly as a result of epigenetic modifications causing a change in chromatin structure. A general alteration of the genome architecture of the neo-Y has occurred within only 1 MY in D. miranda. In particular, the neo-Y of this species is characterized by massive decay in gene function, including the fixation of stop codons and frame shift mutations, large deletions and a general accumulation of repetitive transposable element DNA. The loss of gene function is accompanied by the beginning of heterochromatinization of the neo-Y. After 15 MY, almost no sequence similarity between the neo-Y and its former homolog can be detected, and the neo-Y has become entirely heterochromatic. This evolutionary stage has been reached in D. pseudoobscura, and resembles the general architecture of the ancestral Y in Drosophila. Genes on the ancestral Y of Drosophila contain massive introns, filled with repetitive DNA. Y-linked genes in Drosophila, such as ks-1, are characterized by huge introns, filled with repetitive satellite DNA.