Abstract
Transcatheter aortic valve replacement can be an effective, reliable treatment for severe aortic stenosis in surgically high-risk or ineligible patients. However, various sequelae like coronary artery obstruction can occur, not only in the long term, but also immediately after the procedure. We present the case of a 78-year-old woman whose left main coronary artery became obstructed with calculus 2 hours after the transfemoral implantation of an Edwards Sapien XT aortic valve. Despite percutaneous coronary intervention in that artery, the patient died. This case reminds us that early recognition of acute coronary obstruction and prompt intervention are crucial in patients with aortic stenosis who have undergone transcatheter aortic valve replacement.
Keywords: Aortic valve stenosis/therapy, calcinosis/complications, coronary occlusion/etiology, fatal outcome, heart valve prosthesis implantation/adverse effects/methods/mortality, risk assessment
Transcatheter aortic valve replacement (TAVR), also called transcatheter aortic valve implantation (TAVI), is often suitable for surgically high-risk or ineligible patients who have aortic stenosis. Although TAVR is less invasive than surgery, major vascular sequelae and strokes occur more often in TAVR groups than in surgical groups.1,2 Coronary artery obstruction during valve implantation is less frequent but can still be fatal.3 We present the case of a patient whose left main coronary artery (LMCA) became obstructed by native-valve calculus 2 hours after TAVR.
Case Report
In November 2012, a 78-year-old woman, diagnosed 4 years earlier with severe degenerative aortic stenosis, presented with a one-year history of increasingly labored breathing and edema in the legs. She was being treated for hypertension, diabetes mellitus, severe chronic obstructive pulmonary disease, and heart failure (New York Heart Association functional class IV). In addition, she had a large umbilical hernia that could not be operated on because of the high surgical risk. This hernia reduced her respiratory capacity by pressing on the lungs through the diaphragm.
Auscultation revealed S3 and S4, a grade 4/6 systolic ejection murmur best heard in the aortic area and spreading through the carotid arteries, thin crepitant crackles extending to the middle zones of the right lung, and decreased breathing sounds in the left lung. The patient had 3+ pretibial edema. An electrocardiogram (ECG) showed atrial fibrillation and nonspecific ST-T changes. A chest radiograph showed a collapsed left lung.
A transthoracic echocardiogram (TTE) revealed severe aortic stenosis (mean gradient, 59 mmHg), moderate tricuspid regurgitation, a systolic pulmonary artery pressure of 48 mmHg, and a left ventricular ejection fraction of 0.65. The patient's calculated Society of Thoracic Surgeons risk score was 5.5%; her Surgical Replacement and Transcatheter Aortic Valve Implantation score placed her at high risk. Because of her age and comorbid conditions, we decided to perform TAVR and not surgery. A transesophageal echocardiogram (TEE) showed a tricuspid aortic apparatus and bulky calculus on and between the valves (Fig. 1). The aortic annular size was 22 mm. Multislice computed tomograms showed no tortuosity or calcification of the iliofemoral arteries but revealed severe calculus on the aortic valve; the distance between the aortic annulus and LMCA was 13 mm (Fig. 2). Coronary angiograms revealed normal coronary arteries.
Fig. 1.
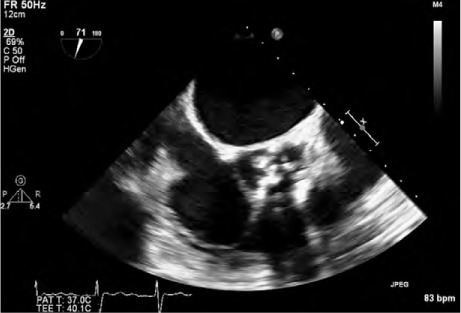
Transesophageal echocardiogram shows calculus on the tricuspid aortic valve.
Fig. 2.
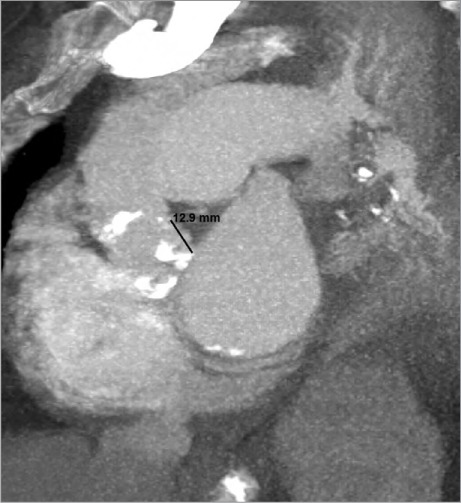
Coronal cardiac computed tomogram shows the calcific aortic valve and the 13-mm distance between the aortic annulus and the left main coronary artery.
In the catheterization laboratory, the patient was placed under general anesthesia. With use of TEE guidance and rapid temporary pacing, we performed aortic balloon valvuloplasty and transfemoral implantation of a 26-mm Edwards Sapien XT Transcatheter Heart Valve (Edwards Lifesciences Corporation; Irvine, Calif). Afterwards, TEE showed appropriate positioning of the prosthetic valve, mild paravalvular aortic regurgitation, and a mean gradient of 7 mmHg. Aortic arch angiograms under fluoroscopic guidance showed patency of the right and left coronary arteries nonselectively and mild aortic regurgitation (Fig. 3). The procedure was considered to be successful. A Prostar XL® Percutaneous Vascular Surgical System (Abbott Vascular, a unit of Abbott Laboratories; Redwood City, Calif) was used to close the right femoral artery percutaneously.
Fig. 3.
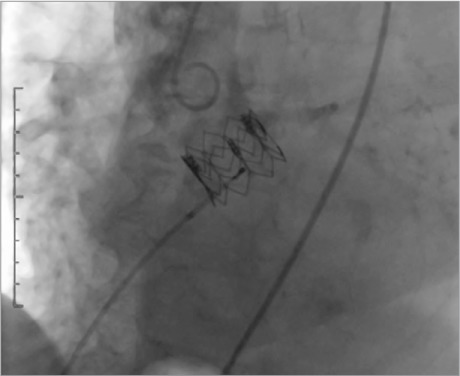
After aortic valve implantation, a coronary angiogram of the aortic arch and valve nonselectively shows open coronary arteries and mild aortic regurgitation.
The intubated patient was hemodynamically stable for 2 hours in the coronary intensive care unit (CICU) until the sudden onset of circulatory collapse (blood pressure, 70/50 mmHg) and bradycardia (heart rate, 42 beats/min). An urgent ECG revealed intraventricular conduction delay and bradycardia. The patient was stabilized with atropine, and the rate of her temporary pacemaker was increased to 80 beats/min. A bedside TTE showed an intact aortic bioprosthesis and a strikingly low left ventricular ejection fraction of 0.15 (vs 0.65 before TAVR). These findings made us suspect acute coronary obstruction. The patient was returned to the catheterization laboratory and went into cardiac arrest. Cardiopulmonary resuscitation (CPR) with intra-arterial monitoring was started. Coronary angiograms during continuous CPR showed total calcific obstruction of the LMCA (Fig. 4). We thought that the superior part of the deployed valve might have caused the coronary obstruction; however, the guiding catheter had been inserted into the LMCA away from the stratum of the aortic bioprosthesis. Predilation was performed with 3.5 and 4 × 12-mm balloons by breaching the calculus on the LMCA with a guidewire, all during CPR. After predilation, a 4 × 12-mm bare-metal stent was deployed, and we observed full patency. The guiding catheter was inserted into the LMCA but not to the interval between the deployed valve strata (Fig. 5). The patient's blood pressure was insufficient despite ensured coronary flow, so the femoral artery was attached to a femoral vein pump with the aid of cardiovascular surgeons, to reduce the workload on the heart. The patient was returned to the CICU, and appropriate medical therapy was applied to provide metabolic balance. During the next 3 hours, intermittent off-pump bypass was performed; however, the necessary hemodynamic values could not be attained. After 7.5 total hours, the patient still had asystole; pump support was withdrawn, and she died.
Fig. 4.
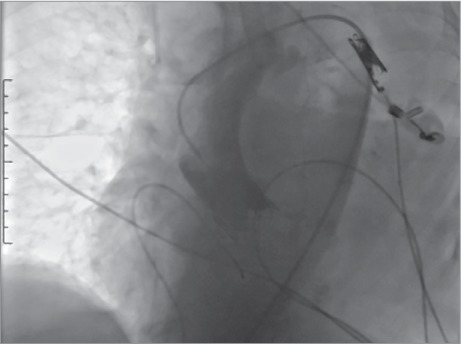
Coronary angiogram shows the aortic arch and calcific obstruction of the left main coronary artery.
Fig. 5.
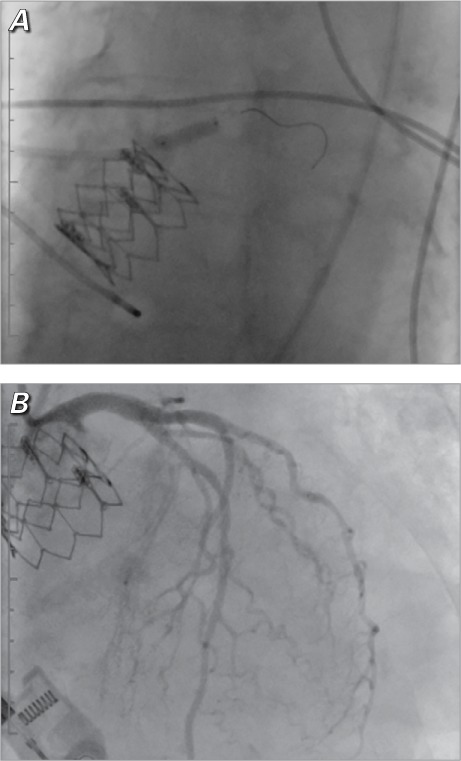
Coronary angiograms show A) the placement of the stent in the left main coronary artery and B) subsequent patency.
Discussion
In large and medium-sized series, the prevalence of coronary artery obstruction after TAVI was 0.6% to 4.1%.4,5 The most frequently identified obstruction is of the LMCA.5 Postprocedural hemodynamic deterioration and newly emerging segmental wall-motion abnormalities can be relevant clues. Investigators have typically reported acute hemodynamic deterioration just after TAVI, while the patient was still in the catheterization laboratory6; however, obstruction can occur either during or a few hours after the procedure. Spiro and associates7 observed obstruction later, after 3.5 hours—in a case similar to ours. Obstructions in the LMCA and right coronary artery after the trans-femoral implantation of an Edwards Sapien valve have been well documented.7–9 Emergent percutaneous coronary intervention (PCI) has been performed as part of resuscitation. Because acute circulatory collapse frequently occurs after LMCA obstruction, manual circulatory support must be initiated immediately. Cardiac arrest occurred in our patient after she was returned to the catheterization laboratory, so we had to perform PCI under continuous, manual CPR. Despite all the problems, she had undergone apparently successful angioplasty and stenting.
Careful evaluation of the aortic valve and aortic root is extremely important in averting catastrophe. In post-TAVR coronary occlusion, risk factors are a narrow aortic annulus, a superficial sinus of Valsalva, less than 12 mm separating the aortic annulus and coronary ostium, previous plaque in the LMCA, large calculus deposits on the aortic valve, and the use of large replacement valves. However, it is unproven that these factors jointly or separately lead to LMCA obstruction, so the cause remains unpredictable and unclear. Before TAVR, 2 important measurements are the distance between the LMCA ostium and the aortic annulus, and the length of the left coronary flap. Despite an appropriate distance between our patient's annulus and LMCA, there was an intensive calcium load over the valves. In addition, the bulky calculus on the aortic cusp, the superficial structure of the sinus of Valsalva, and the 26-mm size of the replacement valve increased the risk of coronary obstruction.
To evaluate coronary obstruction, aortography is typically performed during aortic valvuloplasty and then routinely after TAVR.4 The position of the prosthetic valve is a concern: once implanted, the currently available devices cannot be repositioned.4 The prosthetic valve should be placed neither too high nor too low: the further above the annulus, the greater the risk of LMCA obstruction. In our patient, aortograms during valvuloplasty and after TAVR showed a patent LMCA.
As a result of our experience, we wish to emphasize that taking the utmost care is crucial during procedures in similar patients. Coronary obstruction should be viewed as a primary danger in patients who present with acute hemodynamic deterioration early or late after TAVR. Routine aortography, selective coronary angiography, and prompt intervention can save lives if coronary obstruction is detected.
References
- 1.Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32(2):148–57. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stabile E, Sorropago G, Cioppa A, Cota L, Agrusta M, Lucchetti V, Rubino P. Acute left main obstructions following TAVI. EuroIntervention. 2010;6(1):100–5. [PubMed] [Google Scholar]
- 3.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113(6):842–50. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122(1):62–9. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 5.Bartorelli AL, Andreini D, Sisillo E, Tamborini G, Fusari M, Biglioli P. Left main coronary artery occlusion after percutaneous aortic valve implantation. Ann Thorac Surg. 2010;89(3):953–5. doi: 10.1016/j.athoracsur.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Saia F, Marrozzini C, Marzocchi A. Displacement of calcium nodules of the native valve as a possible cause of left main occlusion following transcatheter aortic valve implantation. J Invasive Cardiol. 2011;23(5):E106–9. [PubMed] [Google Scholar]
- 7.Spiro J, Nadeem A, Doshi SN. Delayed left main stem obstruction following successful TAVI with an Edwards SAPIEN XT valve: successful resuscitation and percutaneous coronary intervention using a non-invasive automated chest compression device (AutoPulse) J Invasive Cardiol. 2012;24(5):224–8. [PubMed] [Google Scholar]
- 8.Kapadia SR, Svensson L, Tuzcu EM. Successful percutaneous management of left main trunk occlusion during percutaneous aortic valve replacement. Catheter Cardiovasc Interv. 2009;73(7):966–72. doi: 10.1002/ccd.21867. [DOI] [PubMed] [Google Scholar]
- 9.Gogas BD, Zacharoulis AA, Antoniadis AG. Acute coronary occlusion following TAVI. Catheter Cardiovasc Interv. 2011;77(3):435–8. doi: 10.1002/ccd.22808. [DOI] [PubMed] [Google Scholar]


