Figure 1.
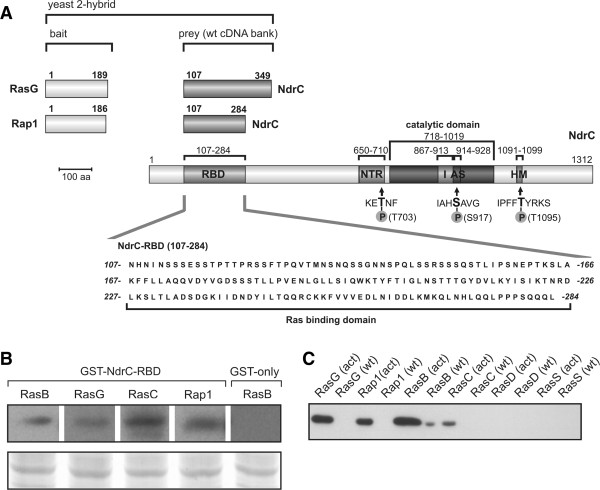
NdrC interacts with Ras proteins. A. In yeast two-hybrid experiments with various activated Ras proteins as bait, NdrC was revealed as a strong interactor of RasG and Rap1. Domain organization of NdrC, and mapping of the Ras binding domain (RBD, aa 107–284), NTR (N-terminal regulatory domain, aa 650–710), phosphorylation site (T703), catalytic domain (kinase domain aa 718–1019, subdomains I-X), I (insert, aa 867–913), AS (activation segment, aa 914–928; regulatory phosphorylation site S917), HM (hydrophobic motif aa 1092–1099; phosphorylation site T1095). B. Binding of GST-NdrC-RBD to Ras proteins in vivo. The RBD of NdrC was used to detect Ras proteins in Dictyostelium cell lysates as described in Methods. The bound material was analyzed by Western blotting using antibodies specific to RasB, RasG, RasC and Rap1 (upper panel). GST-only was tested in all pull-down experiments, and consistently there was no binding (here shown only for RasB). Equal sample loading was verified by staining of a duplicate gel with Coomassie Blue (lower panel); only the range of the strongest band is shown. C. Representative Western blot showing the pull-down of His-tagged Ras proteins by GST-tagged NdrC-RBD. Recombinant His-tagged Ras proteins (constitutively GTP- or GDP-bound) were allowed to bind in vitro to GST-tagged bacterially expressed NdrC-RBD. For details of the quantitative assay please see Methods. The amount of bound Ras proteins was detected by Western blotting using an anti-His Tag antibody.
