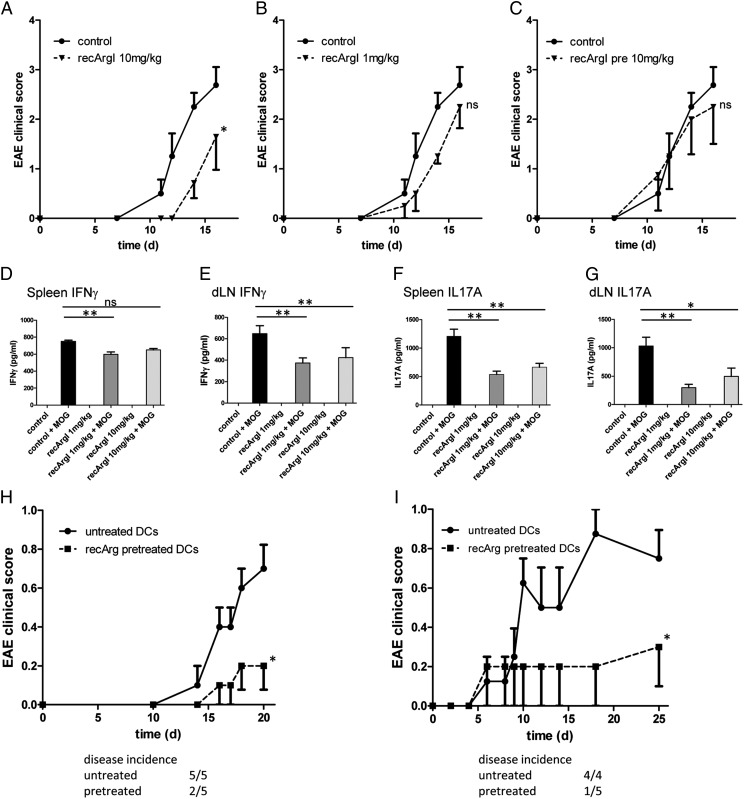
FIGURE 5.
Treatment with recombinant Arginase I at early stages of disease development protects mice from development of EAE. (A–C) Disease development as indicated by clinical score in recArgI-treated versus control WT mice. (D–G) Cytokine secretion after ex vivo restimulation with MOG35–55 peptide of spleen cells and draining inguinal lymph node (dLN) cells from MOG35–55–immunized mice belonging to groups 1 and 2 (see Table I). (H) Clinical score of 2D2 TCR tg mice after transfer of recArgI pretreated or untreated BMDCs, which were pulsed with MOG35–55 peptide (30 μg/ml) and LPS (100 ng/ml). (I) Disease progression of 2D2 TCR tg mice receiving recArgI pretreated or untreated DCs pulsed with MOG35–55 peptide (50 μg/ml) and primed with LPS (100 ng/ml) and additional administration of CFA and pertussis toxin. *p < 0.05 in the EAE, (two-way ANOVA between the groups as indicated) *p < 0.05, **p < 0.01 for the evaluation of cytokine release. ns, not significant. Data are representative of experiments with four to eight mice per group (error bars represent SD).