Figure 1.
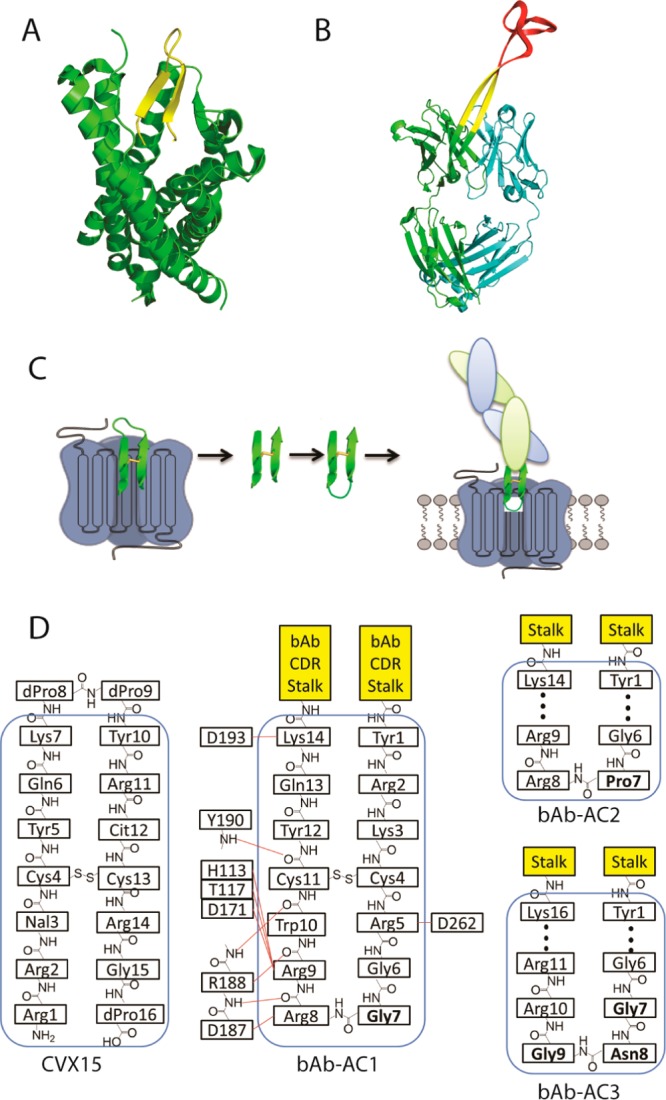
Antibody design. (A) Crystal structure of CXCR4 (green) in complex with a β-hairpin peptide antagonist CVX15 (yellow) (PDB code 3OE0). (B) Crystal structure of bovine antibody BLV1H12 (PDB code 4K3D) shows a disulfide cross-linked “knob” domain (red) grafted onto a solvent-exposed β-strand “stalk” (yellow). (C) A cartoon representation of the anti-CXCR4 antibody design. The loop region of the β-hairpin that resides outside the binding pocket of CXCR4 (blue) is removed, and the antiparallel β-strand region (green) is reconnected by selected β-turns to generate an inverted β-hairpin that is fused to the knob domain truncated bovine antibody scaffold. (D) A schematic representation of CVX15 and the engineered CDRs with β-turn promoting residues highlighted in bold. Potential interactions of bAb-AC1 with the CXCR4 ligand-binding pocket (blue box) are depicted on the basis of an analysis of the CXCR4-CVX15 complex.10
