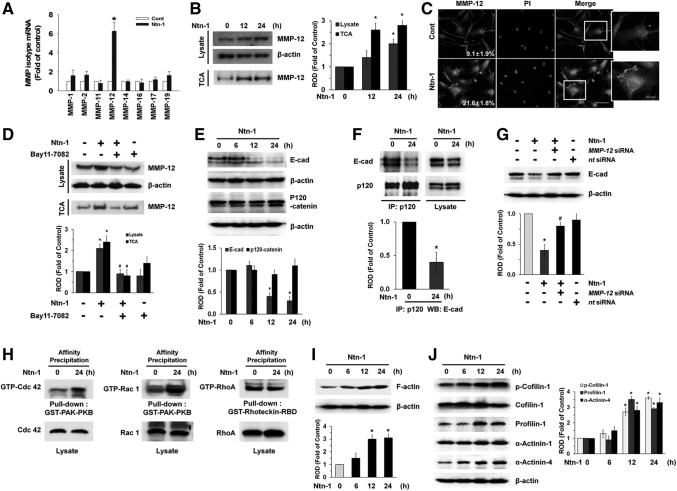
FIG. 4.
Effect of Ntn-1 on the degradation of E-cadherin (E-cad) via MMP-12. (A) Cells were treated with 50 ng/mL Ntn-1 for 12 h. The mRNA expression of MMP family was measured in cells using real-time polymerase chain reaction as described in the Materials and Methods section. Error bars represent the mean±SE from four independent experiments involving triplicates. *P<0.01 versus control. (B) Cells were treated with 50 ng/mL Ntn-1 for 12 or 24 h. Proteins from cell lysates and protein precipitated by TCA in medium were extracted and blotted with MMP-12 antibody. Error bars represent mean±SE of four independent experiments for each condition determined from densitometry relative to β-actin. *P<0.05 versus 0 h. (C) The cells were treated with Ntn-1 (50 ng/mL) for 24 h. MMP-12 was detected by immunostaining with MMP-12 antibody. The expression of MMP-12 was detected by immunostaining with MMP-12 antibody, and quantified by using Image J program. The quantification data of immunofluorescence images were presented as mean±SE of three independent experiments. Scale bars represent 100 μm (magnification, ×400). PI was used for nuclear counterstaining. (D) The cells were pretreated NF-κB inhibitor Bay 11–7082 (10 μM) for 30 min prior to Ntn-1 exposure for 24 h. Proteins from cell lysates and protein precipitated by TCA in medium were extracted and blotted with MMP-12 antibody. Error bars represent mean±SE of four independent experiments for each condition determined from densitometry relative to β-actin. *P<0.01 versus vehicle. #P<0.01 versus Ntn-1 alone. (E) The cells were incubated in the presence of Ntn-1 (50 ng/mL) for various times (0–24 min) and then harvested. Total protein was extracted and blotted with E-cad and p120-catenin antibodies. Error bars represent the mean±SE of four independent experiments for each condition determined from densitometry relative to β-actin. *P<0.01 versus 0 h. (F) The cells were incubated in the presence of Ntn-1 (50 ng/mL) for 24 h and then harvested. p120-catenin was immunoprecipitated with an anti-p120-catenin antibody, and co-immunoprecipitated E-cad were detected by using anti-E-cad antibody (left panels). Expression of p120-catenin and E-cad in total cell lysates is shown in the right panels. n=4 Error bars represent the mean±SE of four independent experiments for each condition determined from densitometry relative p120-catenin binding co-immunoprecipitated with p120-catenin antibody. *P<0.01 versus 0 h. (G) Cells were transfected for 24 h with MMP-12 siRNA (200 pM) using Hyperfectamine before Ntn-1 (50 ng/mL) exposure for 24 h. Nontargeting (nt) control siRNA was used as a negative control (200 pM). Total protein was extracted and blotted with E-cad antibody. Error bars represent the mean±SE of three independent experiments for each condition determined from densitometry relative to β-actin. *P<0.01 versus vehicle. #P<0.05 versus Ntn-1 alone. (H) Cells were treated with Ntn-1 for 24 h, and the lysates (400 μg) were incubated with agarose beads coupled with GST-PAK-PBD or GST-Rhotekin-RBD. The bound activated GTP-Cdc42, GTP-Rac1, and RhoA were resolved by SDS-PAGE, transferred, and blotted using an anti- Cdc42, anti-Rac1, and anti-RhoA antibodies to determine the extent of the activation of Cdc42, Rac1, and RhoA. Total Cdc42, Rac1, and RhoA was determined using lysates (bottom panels). Representative results from three independent experiments are shown. (I, J) The cells were incubated in the presence of Ntn-1 (50 ng/mL) for various times (0–24 min) and then harvested. Total protein was extracted and blotted with F-actin, phospho-Cofilin-1, Cofilin-1, Profilin-1, α-Actinin-1, and α-Actinin-4 antibodies. Error bars represent mean±SE of four independent experiments for each condition determined from densitometry relative to β-actin. *P<0.01 versus 0 h. F-actin, filamentous actin; IP, immunoprecipitation; MMP, matrix metalloproteinase; ROD, relative optical density; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; TCA, trichloroethanoic acid.