Figure 7. Diagram to elucidate a possible mechanism of how fluoride activates the Gαq signaling pathway, in turn stabilizing SATB1 protein in secretory ameloblasts.
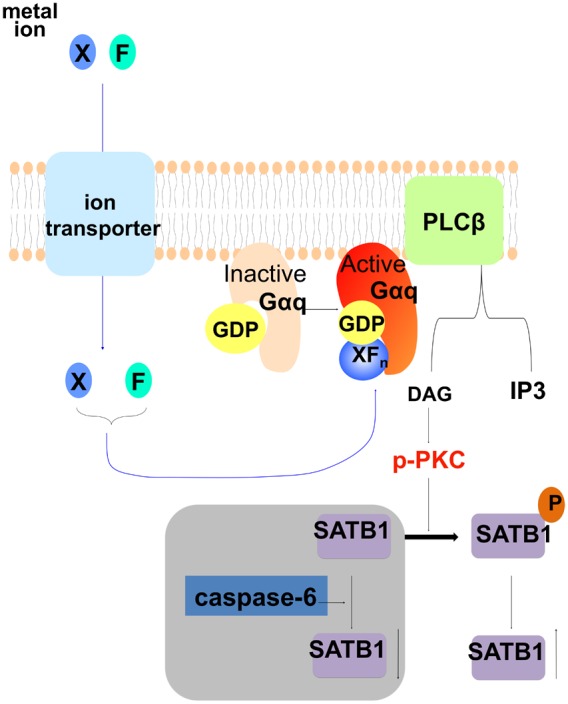
This possible mechanism suggests that fluoride can combine with other ions (X) to mimic the chemical structure of γ-phosphate. Upon entering the cells, ion/fluoride complex (XFn) (such as AlF4) can bind to GDP, together to activate Gαq protein in ameloblasts, which in turn activates phospholipase C β (PLCβ) that cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Increased DAG sequentially activates PKC, which can then phosphorylate SATB1. SATB1 can be hydrolyzed by caspase-6, while phosphorylation prevents SATB1 from hydrolyzing by caspase-6, resulting in an apparent relative increase in SATB1 in cells.
