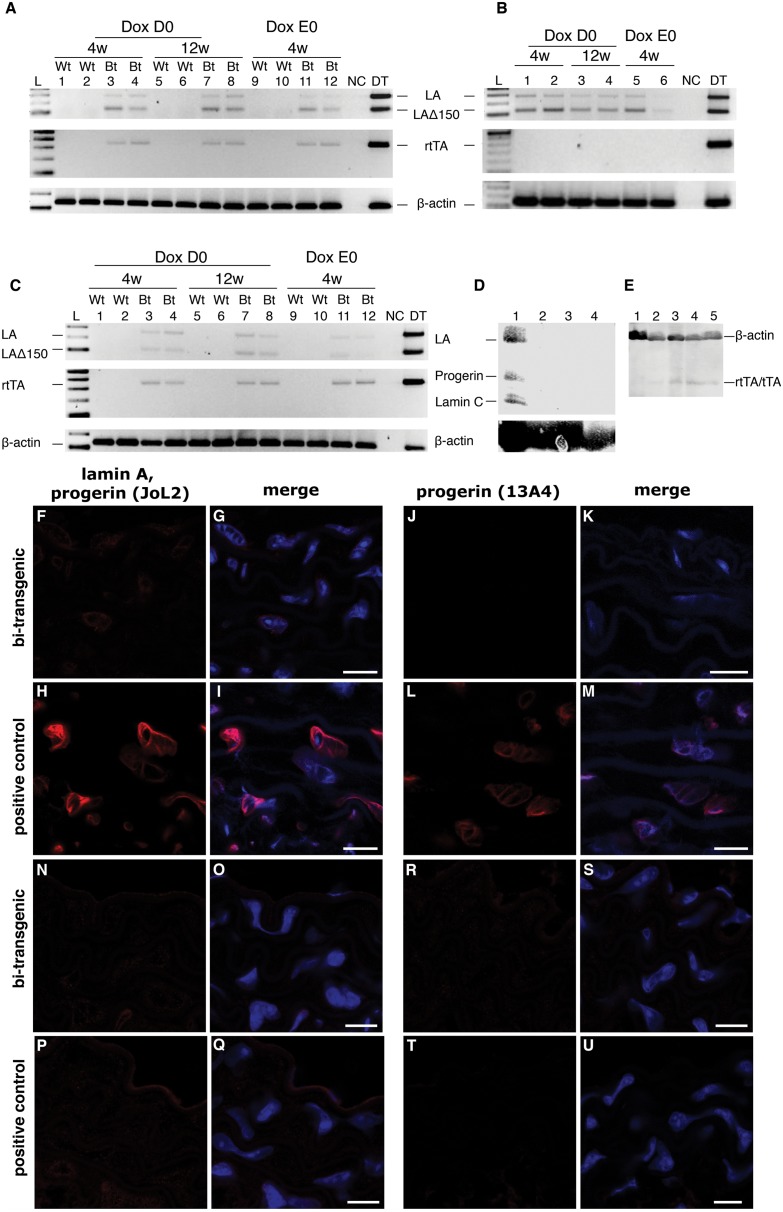
Figure 1. Low levels of transgene expression in the aortic arch.
(A–C) RT-PCR using mRNA from the aortic arch showed very weak amplification products for human lamin A, lamin Adel150 and the reverse transactivator after 35 cycles of PCR. (A, C) RT-PCR on samples from bi-transgenic mice encoding both the reverse transactivator (sm22α-rtTA+) and the lamin A minigene (tetop-LAG608G+) that were supplied with doxycycline from the date of birth until postnatal week 4 (Dox D0, lanes 3–4) or 12 (Dox D0, lanes 7–8), or supplied with dox during embryogenesis and postnatally for 4 weeks (Dox E0, lanes 11–12). (A) C57BL/6J genetic background. (C) C57BL/6J; FVB/NCrl mixed genetic background. (B) RT-PCR for human lamin A and lamin Adel150 in samples from transactivator negative control animals (tetop-LAG608G+; sm22α-rtTA−, lanes 1–6) supplied with doxycycline from the date of birth until postnatal week 4 (Dox D0, lanes 1 and 2, C57BL/6J and C57BL/6J; FVB/NCrl genetic background, respectively) or week 12 (Dox D0, lanes 3 and 4, C57BL/6J and C57BL/6J; FVB/NCrl genetic background, respectively), or that were supplied with doxycycline during embryogenesis and postnatally for 4 weeks (Dox E0, lanes 5 and 6, C57BL/6J and C57BL/6J; FVB/NCrl genetic background, respectively). Bt, bi-transgenic. Wt, wild-type. NC, control with no template. DT, sample from a different transactivator strain was used as a control for the PCR assay and showed amplification for human lamin A and lamin Adel150 with cDNA from the bone of bi-transgenic tetop-LAG608G+; Sp7-tTA+ mice [18]. Genomic DNA from a tetop-LAG608G+; sm22α-rtTA+ bi-transgenic animal was used as a positive control for the amplification of the reverse transactivator (350 base pair product) [15]. The RT-PCR results for β-actin served as a control. (D) Western blot analysis on protein extracts from pooled aortic regions from bi-transgenic tetop-LAG608G+; sm22α-rtTA+ (lane 2) and tetop-LAG608G+; NSE-tTA+ (lane 3) animals did not show transgenic expression of human lamin A and progerin. A single transgenic tetop-LAG608G−; sm22α-rtTA+ animal carrying only the transactivator was used as a negative control (lane 4). Protein extract from HGPS patient cell line AG11513A was used as positive control (lane 1). (E) Western blot analysis on protein extracts from pooled aortic regions from bi-transgenic tetop-LAG608G+; sm22α-rtTA+ (lanes 2 and 5) and single transgenic animals, tetop-LAG608G−; NSE-tTA+ (lane 3) and tetop-LAG608G−; sm22α-rtTA+ (lane 4). Protein extracts from wild-type tissue, tetop-LAG608G−; NSE-tTA−, was used as a negative control (lane 1). (F–G and J–K) Very few transgene positive cells, <1%, were detected in the aortic arches of bi-transgenic animals at postnatal week 12 using antibodies specific for human lamin A/C and progerin (JoL2) (F–G), and human progerin (13A4) (J–K). (H–I and L–M) Positive staining was obtained using the same antibodies, on sections of the aortic arch from tetop-LAG608G+; NSE-tTA+ bi-transgenic mice [17]. (N–O and R–S) Very few transgene protein positive cells, <1%, were detected in the aortic arches of adult bi-transgenic animals, not supplied with doxycycline for the last 4 weeks prior to sacrifice, using antibodies for human lamin A/C and progerin (JoL2) (N–O), and human progerin (13A4) (R–S). (P–Q and T–U) Almost no positive staining was obtained using the same antibodies, on sections of the aortic arch from tetop-LAG608G+; NSE-tTA+ bi-transgenic mice supplied with doxycycline for 3 weeks (indicating a significant down-regulation of the transgenic expression with the doxycycline supplement). G, I, O, Q: merge of the transgenic lamin A and progerin with DAPI fluorescence signals. K, M, S, U: merge of the progerin and DAPI fluorescence signals. Scale bars: 10 µm.