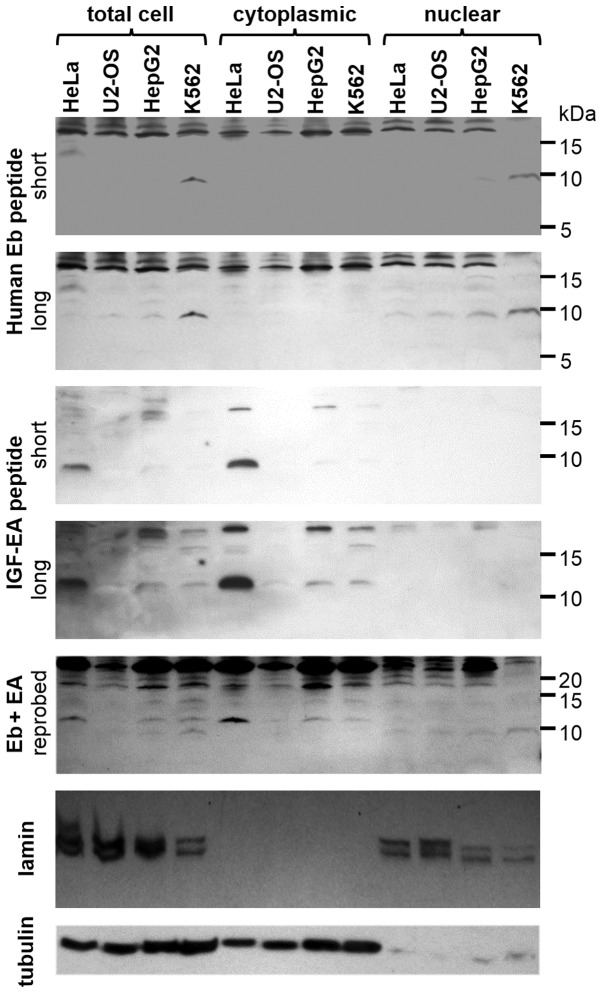
Figure 4.
Immunoblotting showing endogenous IGF isoforms in human cancer cells. Sixty micrograms of protein were resolved in each lane. A 9 kD band is present in total cell extract and nuclear fraction from K562 cells and, to a smaller extent, in HepG2 when probed with anti-Eb peptide antibody on the first membrane (short exposure to X-ray film). After longer exposure, this band was also slightly visible in the other two cell lines used in this study (HeLa, U2OS), second panel. It never appeared in cytoplasmic fraction of these cells. A 11–12 kD band was detected when the same membrane was probed with anti-EA antibody, corresponding to human pro-IGF-1A (panel 3). However, this band was evident only in total cell extract and cytoplasmic fraction and never present in the nuclear one (panel 4). Also, another blotting is shown (panel 5), the same membrane already probed with anti-Eb antibody was stripped and reprobed with anti EA-peptide antibody. A merge of both anti-Eb and anti-EA pattern can be observed and a molecular mass difference of 2–3 kD between human Eb-peptide (9 kD) and pro-IGF-1A (11–12 kD). For confirmation of cellular fractionation, 2 different antigens are shown: lamin (nuclear marker), tubulin (cytoplasmic marker) (panels 6 and 7). IGF, insulin-like growth factor.