Fig. 1.
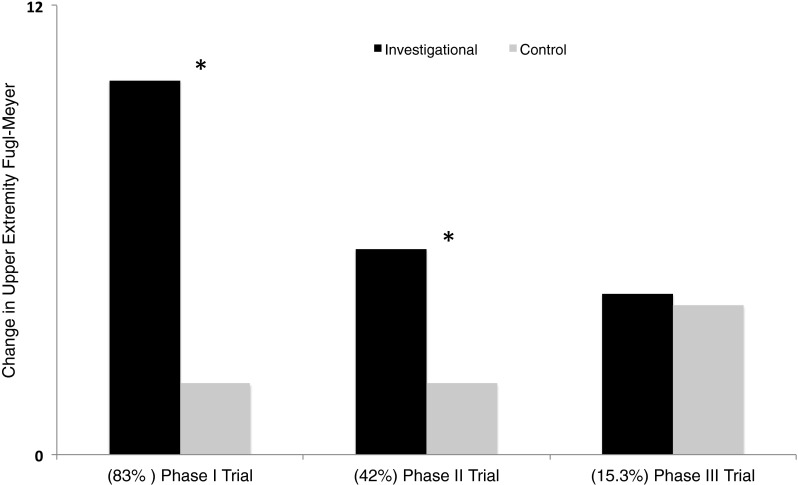
Representation of data from early clinical trials and latest phase III clinical trial of invasive cortical stimulation in stroke. All trials compared an investigational group that received stimulation of peri-lesional M1 during rehabilitation to a control group that received rehabilitation alone. Black bars represent percentage of patients in investigational group who achieved a clinically meaningful change in upper extremity Fugl–Meyer score; gray bars show percentage of patients in the control group who achieved such a meaningful effect. In parentheses below, we list the percentage of patients in the investigational group in respective trials who were deemed to possess functioning corticospinal tracts. These values are derived from Brown et al. [20] (phase I), Levy et al. [18], Huang et al. [17] (phase II), and Nouri and Cramer [25] (phase III trial). Asterisk (*) denotes reported statistical significance between investigational and control groups. One can note a qualitatively inverse relationship between advantage of invasive cortical stimulation and stage of clinical trials. An important reason for such a relation may be the decreasing percentage of patients with functioning corticospinal tracts from early to late clinical trials
