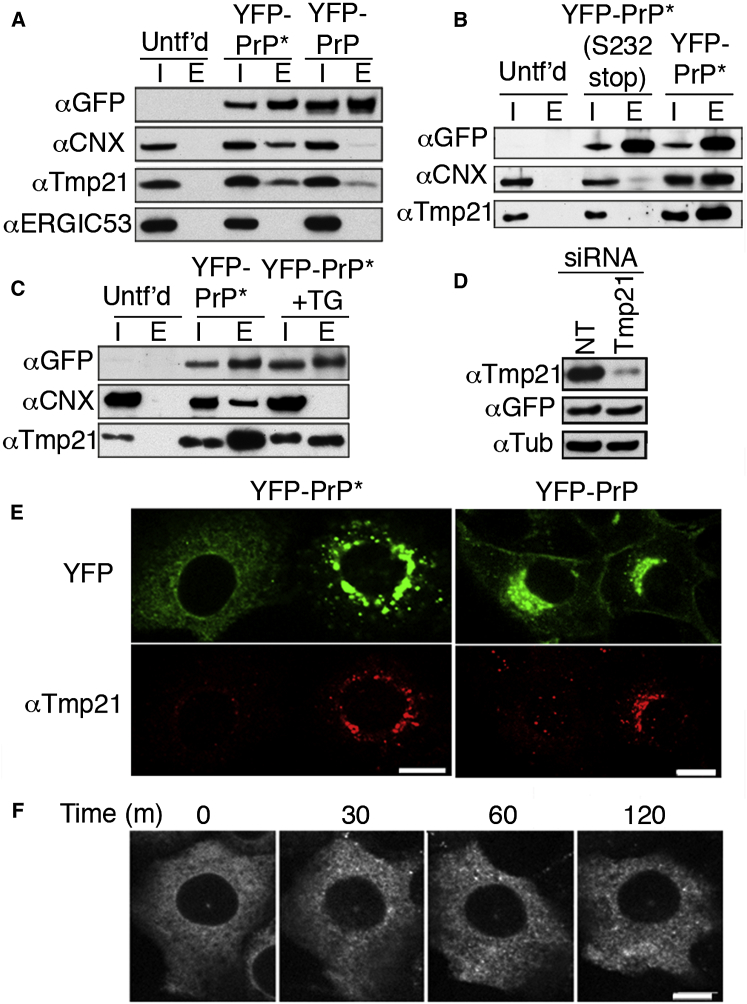
Figure 5.
PrP∗ Releases from Calnexin upon ER Stress for Tmp21-Dependent Export
(A–C) Cells stably expressing the indicated PrP constructs were subjected to GFP pull-downs and input (I) and eluate (E) fractions analyzed by western blot for the indicated proteins, including calnexin (CNX), Tmp21, and p58/ERGIC53. Untransfected cells (Untf’d) served as a negative control. In (A), YFP-PrP-expressing cells were treated with BFA for 3 hr to retain newly synthesized YFP-PrP in the ER. In (C), TG treatment, where indicated, was for 30 min.
(D) Western blot of YFP-PrP∗ cells treated with nontargeting (NT) or Tmp21 siRNA probed for Tmp21, YFP-PrP∗, or tubulin.
(E) Cells expressing either YFP-PrP∗ (left) or YFP-PrP (right) were subjected to siRNA treatment against Tmp21 and analyzed for YFP localization and Tmp21 immunofluorescence. Each panel shows two adjacent cells in the same field of view where one cell was depleted for Tmp21 and the other cell was not. YFP-PrP∗ cells were treated with TG for 30 min prior to fixation, whereas YFP-PrP cells were treated with BFA for 3 hr, released for 60 min, and then fixed. Quantification of multiple fields showed that YFP-PrP∗ was ER localized in all Tmp21-depleted cells (n = 133), but Golgi localized in Tmp21-expressing cells (n = 117). By contrast, YFP-PrP was detected in vesicular compartments and the plasma membrane regardless of Tmp21 knockdown (n = 200).
(F) Time-lapse images after TG treatment of YFP-PrP∗ in a cell knocked down for Tmp21. Identical results were obtained in ∼50% of all cells (n = 112), which is consistent with the proportion of cells successfully knocked down for Tmp21.
Scale bars, 10 μm. See also Figure S4.