Figure S1.
Characterization of YFP-PrP∗ Stress-Induced Degradation, Related to Figure 1
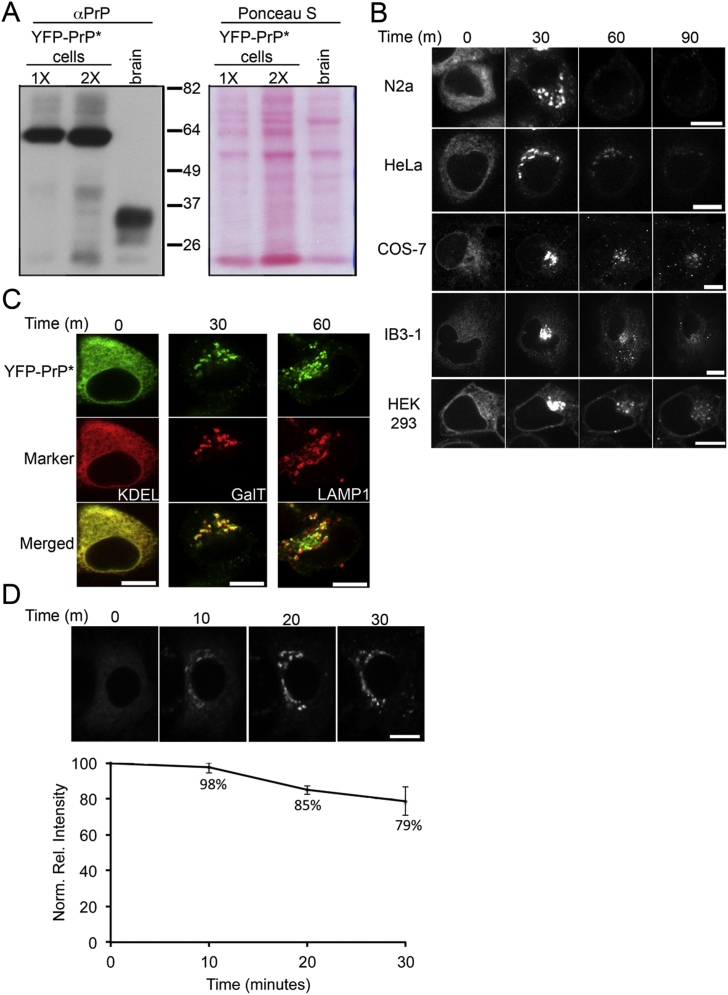
(A) Immunoblot for PrP (using the PrP-A antibody) comparing two different amounts (1X and 2X) of NRK cell lysate stably expressing YFP-PrP∗ with mouse brain homogenate. YFP-PrP∗ appears as a single ∼60 kDa band while endogenous mouse brain PrP appears as a set of ∼28-37 kDa bands. Ponceau S staining of the blot (right panel) reveals that the relative total protein concentration of YFP-PrP∗ cell lysate in the 1X loading lane is similar to mouse brain lysate.
(B) The indicated cell lines were transiently transfected with YFP-PrP∗, and 48 hr later, treated with 0.1 μM thapsigargin (TG). Shown are images at different times after TG addition. Cells analyzed are mouse neuroblastoma (N2a), human cervical cancer (HeLa), African green monkey kidney (COS-7), bronchial epithelial (IB3-1), and human embryonic kidney (HEK293).
(C) N2a cells were co-transfected with YFP-PrP∗ and the indicated organelle markers and, 48 hr later, treated with TG. Representative images of cells at different time points after TG treatment are shown. CFP-KDEL (KDEL) marks the ER. Resident Golgi protein galactosyltransferase T-CFP (GalT) marks the Golgi. LAMP1-CFP (LAMP1) marks the lysosomes. For the 60 min time point only, 125 μM leupeptin was used to inhibit lysosomal degradation so that YFP-PrP∗ could be visualized inside lysosomes.
(D) Comparison of total YFP-PrP∗ fluorescence levels before and after stress-induced relocalization to post-ER compartments. Cells stably expressing YFP-PrP∗ were treated with TG and imaged at 10 min increments within the dynamic range of the camera. Images of a representative cell are shown above a graph quantifying 5 cells (mean ± standard error). By 30 min after acute ER stress, when all the detectable YFP-PrP∗ has left the ER and entered the secretory pathway, there is at least 79% of total starting fluorescence.
Scale bars, 10 μm