Fig. 2.
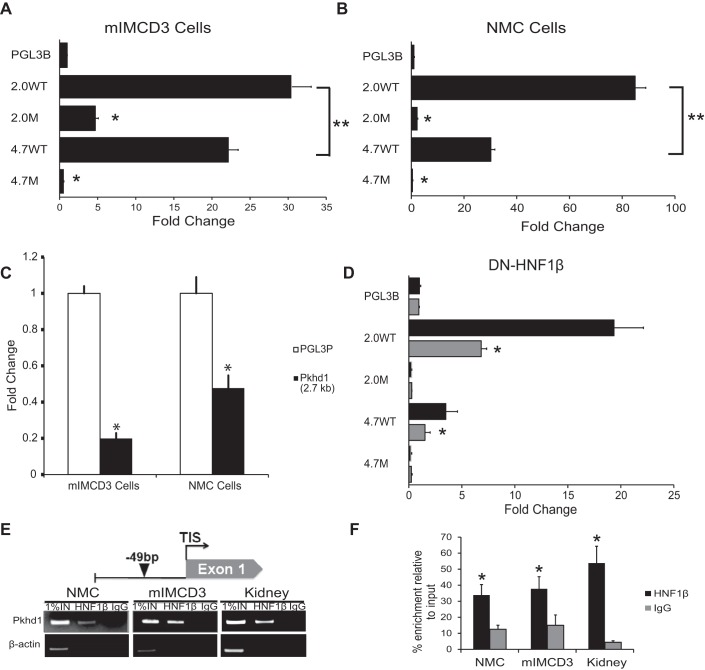
Pkhd1 promoter activity in transfected cells. A and B: mIMCD3 renal epithelial cells (A) and normal mouse cholangiocytes (NMCs; B) were transfected with equimolar amounts of the indicated luciferase reporter plasmids. Luciferase activity was measured after 48 h and normalized to empty pGL3-Basic (pGL3B). Data are means ± SE of six independent experiments. Both 2.0- and 4.7-kb genomic fragments drove transcription in mIMCD3 cells and NMCs. The 2.0-kb fragment had significantly greater activity than the 4.7-kb fragment (**P < 0.05). Mutation of the HNF-1β-binding site inhibited Pkhd1 promoter activity in both cell types. *P < 0.001 compared with wild type. C: mIMCD3 cells or NMCs were transfected with the plasmid Pkhd1(2.7 kb) or empty pGL3-Promoter (pGL3P), and luciferase activity was measured after 48 h. The 2.7-kb fragment inhibited promoter activity in both cell types. *P < 0.001 compared with pGL3-Promoter. D: 53A cells were transfected with the indicated reporter plasmids, and expression of dominant negative mutant HNF-1β (DN-HNF-1β) was induced with 10 nM mifepristone (Mif; shaded bars). Uninduced cells received vehicle [ethanol (EtOH); solid bars]. Luciferase activity was measured after 48 h and normalized to empty pGL3-Basic (pGL3B). *P < 0.05 compared with uninduced cells. E: schematic diagram of the mouse Pkhd1 promoter (top) showing the HNF-1β-binding site at −49 bp identified by chromatin immunoprecipitation (ChIP)-seq (arrowhead). ChIP (bottom) showed occupancy of the sites by endogenous HNF-1β in chromatin from NMCs, mIMCD3 cells, and the mouse kidney on postnatal day 28 (P28). Immunoprecipitation with IgG was used as a negative control, and the first lanes contained 1% of the input DNA. F: histogram showing densitometric analysis of three independent experiments. *P < 0.05 compared with control IgG.