Fig. 7.
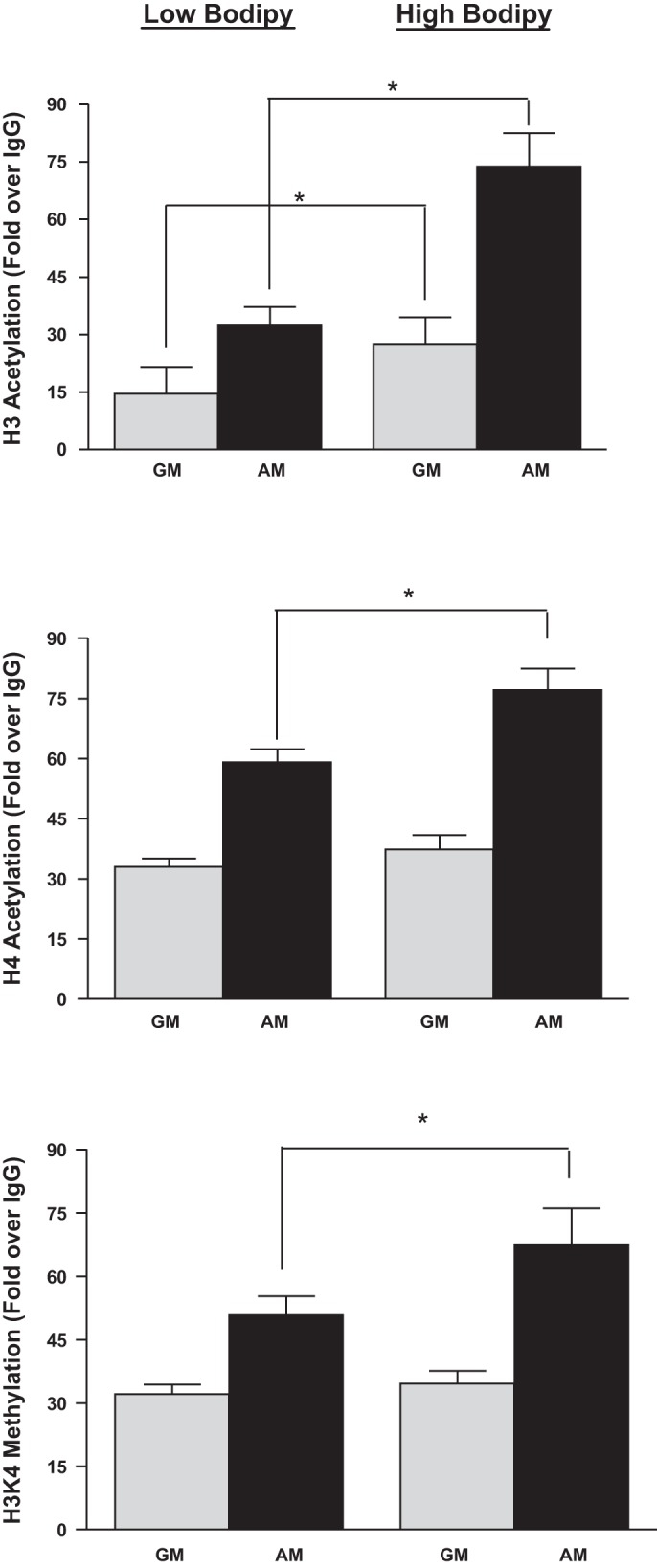
high-BODIPY cells exhibit higher histone H3 acetylation levels than low-BODIPY cells after adipocyte differentiation. Chromatin immunoprecipitation (ChIP) of indicated 3T3-L1 cell populations with antibodies targeted at acetylated histone H3, acetylated H4, and methylated H3K4 on the Pparγ gene promoter. Undifferentiated cells were cultured in GM and differentiated cells in AM. These modifications on the GAPDH promoter, by contrast, remained unchanged in all cell population conditions (data not shown).Values are means ± SD of 3 independent experiments. Levels of H3 and H4 acetylation and H3K4 methylation were higher in both high- and low-BODIPY populations in AM vs. GM (P < 0.02 for H3 acetylation; P < 0.01 for H4 acetylation; P < 0.03 for H3K4 trimethylation). H3 and H4 acetylation were higher in high- vs. low-BODIPY populations in AM (P < 0.02) but not in H3K4 trimethylation (P > 0.05). Importantly, levels of H4 acetylation and H3K4 methylation in cells in GM were not different in high- and low-BODIPY populations (P > 0.05) but were higher in H3 acetylation in chromatin from cells from high- vs. low-BODIPY content populations in GM (*P < 0.01).
