Fig. 2. Ca2+-sensitivity is decreased upon pseudo-phosphorylation of Ser42/44 in cardiomyocytes from failing human hearts.
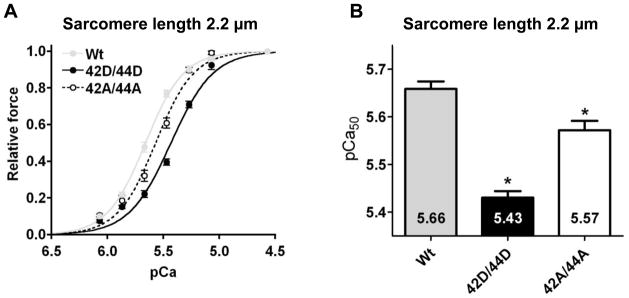
Myofilament force development measured at a sarcomere length of 2.2 μm at various [Ca2+] in permeabilized cardiomyocytes from idiopathic dilated cardiomyopathy (IDCM) patients in which endogenous troponin complex was partially exchanged (65.9±1.0%) with 1 mg/mL of the recombinant myc-tag labeled troponin complexes (12 cardiomyocytes from 3 IDCM hearts per group). A. Compared to wild-type cTnI (Wt), the relative force plotted against pCa demonstrates a shift of the curve to the right upon exchange with pseudo-phosphorylated cTnI at Ser42/44 (42D/44D) and upon exchange with cTnI pseudo-dephosphorylated at Ser42/44 (42A/44A). B. Compared to Wt, Ca2+-sensitivity derived from the midpoint of the force–pCa relationship (pCa50) was significantly decreased after exchange with 42D/44D and 42A/44A. pCa50 was significantly lower in 42D/44D compared to 42A/44A. *, p<0.05, Wt versus 42D/44D, 42A/44A in post-test Bonferroni analyses of one-way ANOVA.
