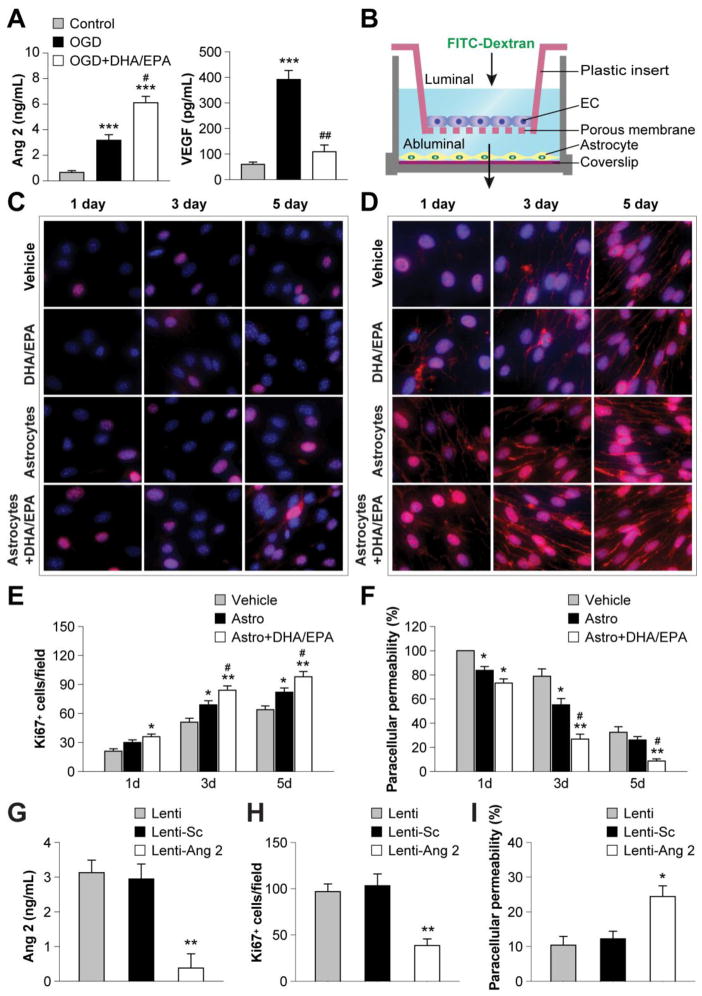
Figure 4. DHA/EPA promote astrocyte angiopoietin 2 release and facilitate endothelial proliferation and barrier formation.
A. Mouse primary astrocytes were treated with DHA/EPA (3μM/7μM) or vehicle for 48 h and then subjected to 60-min OGD. The concentrations of Ang 2 and VEGF in the culture medium were measured quantitatively 24 h after OGD using ELISA. Data are presented as mean ± SEM from three independent experiments, ***p≤0.001 vs. control. #p≤0.05, ##p≤0.01 vs. OGD. B. Illustration of the transwell coculture system allowing cell contact-independent interactions between endothelial cells (ECs) and astrocytes through diffusible factors. Mouse primary ECs were cultured in the luminal chamber of the transwell inserts in the presence or absence of primary astrocytes in the abluminal chamber. Astrocytes grown on a coverslip were treated with DHA/EPA (3μM/7μM) or vehicle for 48 h, followed by 60-min OGD, and then placed into the coculture abluminal chamber. Paracellular permeability of ECs was determined by measuring the diffusion coefficient of FITC-Dextran (40 kDa) from the luminal to the abluminal chamber, and data are expressed as percentage of paracellular permeability in controls. The paracellular permeability of vehicle-treated ECs in the absence of astrocytes at 1 d of coculture was expressed as 100%. C–D. ECs were treated with DHA/EPA (3μM/7μM) or vehicle for 48 h, or cocultured with OGD-treated astrocytes as described above, and then processed for immunostaining. Representative immunofluorescent staining for Ki67 (C) and VE-cadherin (D) in ECs 1–5 d after coculture (red). Cells were counterstained with DAPI (blue) for nuclear labeling. E–F. Immunostained Ki67+ cells were counted as a measure of active cell proliferation (E), and paracellular permeability was calculated as described above (F). Data are presented as mean ± SEM, *p≤0.05, **p≤0.01 vs. vehicle. #p≤0.05 vs. EC+astrocyte (Astro) from 3–4 independent experiments for each condition. G–I. Primary astrocytes were transfected with an empty vector (Lenti), vector with scrambled control sequence (Lenti-Sc), or lentiviral vectors containing shRNA targeting Ang 2 (Lenti-Ang 2). Cells were treated with lentiviral vectors and DHA/EPA (3μM/7μM) or vehicle for 48 h and then subjected to 60-min OGD. G. The release of Ang 2 from astrocytes was measured by ELISA 1 d after OGD. H–I. Transfected astrocytes were cocultured with ECs as described above. H. Ki67+ ECs 3 d after coculture. I. EC paracellular permeability 5 d after coculture. Data are presented as mean ± SEM, *p≤<0.05, **p≤0.01 vs. Lenti from 3–4 independent experiments for each condition.