Significance
Shoot gravitropism is a key determinant of tiller angle, one of the most important factors that affect ideal plant architecture and grain yield of cereal crops. Strigolactones (SLs) are newly identified plant hormones that play diverse roles in plant growth and development. In this study, we provide compelling evidences that SLs are involved in shoot gravitropism, showing that SLs attenuate shoot gravitropism by inhibiting auxin biosynthesis. Our study uncovers a new role of SLs and suggests a previously unidentified mechanism underlying shoot gravitropism and tiller angle. Based on our study, SLs could be considered an important tool for achieving ideal plant architecture in the future.
Abstract
Tiller angle, a key agronomic trait for achieving ideal plant architecture and increasing grain yield, is regulated mainly by shoot gravitropism. Strigolactones (SLs) are a group of newly identified plant hormones that are essential for shoot branching/rice tillering and have further biological functions as yet undetermined. Through screening for suppressors of lazy1 (sols), a classic rice mutant exhibiting large tiller angle and defective shoot gravitropism, we identified multiple SOLS that are involved in the SL biosynthetic or signaling pathway. We show that SL biosynthetic or signaling mutants can rescue the spreading phenotype of lazy1 (la1) and that SLs can inhibit auxin biosynthesis and attenuate rice shoot gravitropism, mainly by decreasing the local indoleacetic acid content. Although both SLs and LA1 are negative regulators of polar auxin transport, SLs do not alter the lateral auxin transport of shoot base, unlike LA1, which is a positive regulator of lateral auxin transport in rice. Genetic evidence demonstrates that SLs and LA1 participate in regulating shoot gravitropism and tiller angle in distinct genetic pathways. In addition, the SL-mediated shoot gravitropism is conserved in Arabidopsis. Our results disclose a new role of SLs and shed light on a previously unidentified mechanism underlying shoot gravitropism. Our study indicates that SLs could be considered as an important tool to achieve ideal plant architecture in the future.
Plant shoot gravitropism is a process in which plants perceive gravity stimuli and reorient the direction of growth during the plant growth and development. Gravitropism is a dynamic process, including the perception of gravity, transduction of the corresponding information into a biochemical signal, transmission of the biochemical signal to a response site, and organ curvature (1). Genetic studies have disclosed that shoot endodermal cells act as statocytes for shoot gravitropism (2). Amyloplast sedimentation transduces the gravitropic signal and leads to auxin redistribution, resulting in a higher auxin level on the lower side than on the upper side (1, 3). Although auxin redistribution upon gravistimulation plays an important role in shoot gravitropism, the mechanism by which auxin regulates shoot gravitropism is not yet understood.
In rice the tiller angle, which is defined as the angle between the tiller and main culm, plays a key role in determining rice plant architecture and thus grain yield (4). Several genes controlling the tiller angle have been identified previously in rice, including LAZY1 (LA1), TILLER ANGLE CONTROL1 (TAC1), PROSTRATE GROWTH1 (PROG1), and LOOSE PLANT ARCHITECTURE1 (LPA1) (5–9). Among these genes, LA1 and LPA1 are reported to regulate tiller angle through shoot gravitropism. In the la1 mutant, the polar auxin transport (PAT) is enhanced, and the lateral auxin transport is decreased, resulting in a disturbance of auxin asymmetric distribution in the shoot base and therefore the tiller-spreading phenotype of rice plants (5).
Strigolactones (SLs), a group of terpenoid lactones, are newly discovered plant hormones that act as inhibitors of shoot branching in higher plants (10, 11). Previous studies have discovered a series of SL biosynthesis and signaling components, MORE AXILLARY GROWTH (MAX) in Arabidopsis, DWARF (D) in rice, RAMOSUS (RMS) in pea, and DECREASED APICAL DOMINANCE (DAD) in petunia (12). Among SL synthesis components, D27 encodes a β-carotene isomerase that converts all-trans-β-carotene into 9-cis-β-carotene (13, 14). MAX3/RMS5/D17/DAD3 and MAX4/RMS1/D10/DAD1 encode the carotenoid cleavage dioxygenase 7 (CCD7) and CCD8, leading to the formation of carlactone, which is catalyzed further by MAX1/OsMAX1 to yield SL compounds (12, 15, 16). For the SL signaling components, MAX2/RMS4/D3 encodes an F-box protein participating in SL perception (17–19), D14/DAD2 encodes a protein of the α/β-fold hydrolase superfamily, a proposed SL receptor (20–25), and D53 encodes a Clp protease family protein as a repressor (26, 27). Recently, SLs also have been found to be involved in regulating stem secondary growth, leaf sentences, seed germination, root development, and abiotic stress responses (28, 29). In this study, we found that mutants defective in the SL biosynthetic and signaling pathways can rescue the spreading phenotype of la1 and further demonstrated that SLs are involved in rice shoot gravitropism to affect rice tiller angle through an LA1-independent pathway.
Results
Isolation of la1 Suppressors Reveals the Involvement of SLs in Shoot Gravitropism/Rice Tiller Angle.
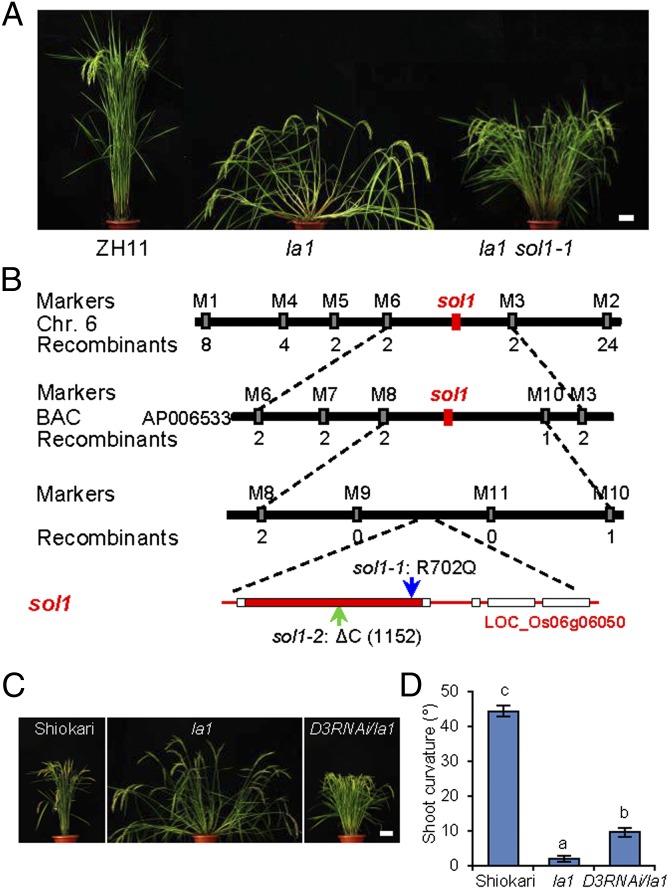
To identify novel components in the LA1-dependent and -independent pathways that regulate shoot gravitropism, we screened for suppressors of la1 (sols) from the ethyl methanesulfonate (EMS)-mutagenized la1 progeny. One of the identified sols, named “sol1,” can mostly rescue the spreading phenotype of la1 (Fig. 1A). The la1 sol1 plant shows a phenotype of reduced plant height and increased tillers gradually standing up after seedling stages (Fig. S1 A–C). From the mutagenized la1 progeny, we found another sol1 allelic mutant, which also is dwarf, high tillering, and erect (Fig. S1 D–F). We therefore designated these mutants “sol1-1” and “sol1-2,” respectively.
Fig. 1.
Cloning and characterization of SOL1. (A) Phenotypes of wild-type (ZH11), la1, and la1 sol1-1 plants at the adult stage. (Scale bar: 10 cm.) (B) Map-based cloning of SOL1. SOL1 was pinpointed in an 80-kb genomic region between molecular markers M8 and M10 and cosegregated with markers M9 and M11 on chromosome 6. Numbers under the markers indicate recombinants. The blue arrow indicates an R702Q amino acid substitution in sol1-1, and the green arrow indicates one base deletion at the first exon of LOC_Os06g06050 that results in a premature translation in sol1-2. The closed box indicates an ORF, and open boxes indicate UTRs. (C) Comparison of tiller angle in the wild-type (Shiokari), la1, and D3RNAi transgenic plants. (Scale bar: 10 cm.) (D) The gravitropic response of D3RNAi/la1 plants at the seedling stage after 24 h gravistimulation. Light-grown seedlings (3 d old) were transferred to darkness and reoriented by 90°. Error bars indicate SEM; n = 10. Means with different letters are significantly different (P < 0.01; ANOVA).
We isolated the SOL1 gene through a map-based cloning approach. In brief, by using 220 F2 plants the SOL1 locus was placed within an 80-kb region between markers M8 and M10, and this 80-kb DNA fragment then was sequenced and compared in la1 and la1 sol1-1. The result showed that one base was replaced at the first exon of LOC_Os06g06050 in la1 sol1-1, resulting in an R702Q amino acid substitution (Fig. 1B), which is conserved across plant species (Fig. S2) and may be involved in substrate recognition (30). In addition, we found that a one-base deletion at the first exon of LOC_Os06g06050 in la1 sol1-2 resulted in a frame shift that leads to a premature translation termination in la1 sol1-2 (Fig. 1B).
Sequence analysis revealed that LOC_Os06g06050 is DWARF3 (D3), an F-box component of the SKP–Cullin–F box (SCF) E3 ubiquitin ligase complex that is essential for SL signal perception (17, 18). To verify the function of D3 in rescuing the la1 phenotype, the D3RNAi plasmid was constructed and transformed into the la1 mutant mediated by Agrobacterium tumefaciens (Fig. S3A). The D3RNAi transgenic plant was much more compact than the la1 plant at the adult stage (Fig. 1C). Accordingly, quantitative RT-PCR (qRT-PCR) analysis showed that D3 expression was reduced significantly in the transgenic plant (Fig. S3B). We also investigated the shoot gravitropic response of D3RNAi transgenic seedlings; their response to gravistimulation was enhanced significantly compared with that of the la1 mutant (Fig. 1D and Fig. S3C), suggesting that the down-regulation of D3 can rescue the spreading phenotype of la1.
Furthermore, we found that other sols display phenotypes similar to sol1 in the EMS-mutagenized la1 progeny. Genetic-linkage analysis and sequencing showed that these sols result from mutations in the SL biosynthetic genes, including D10, D17, and D27 (Fig. S4), suggesting that SLs may play an important role in regulating plant shoot gravitropism.
SLs Attenuate Shoot Gravitropic Response in Rice.
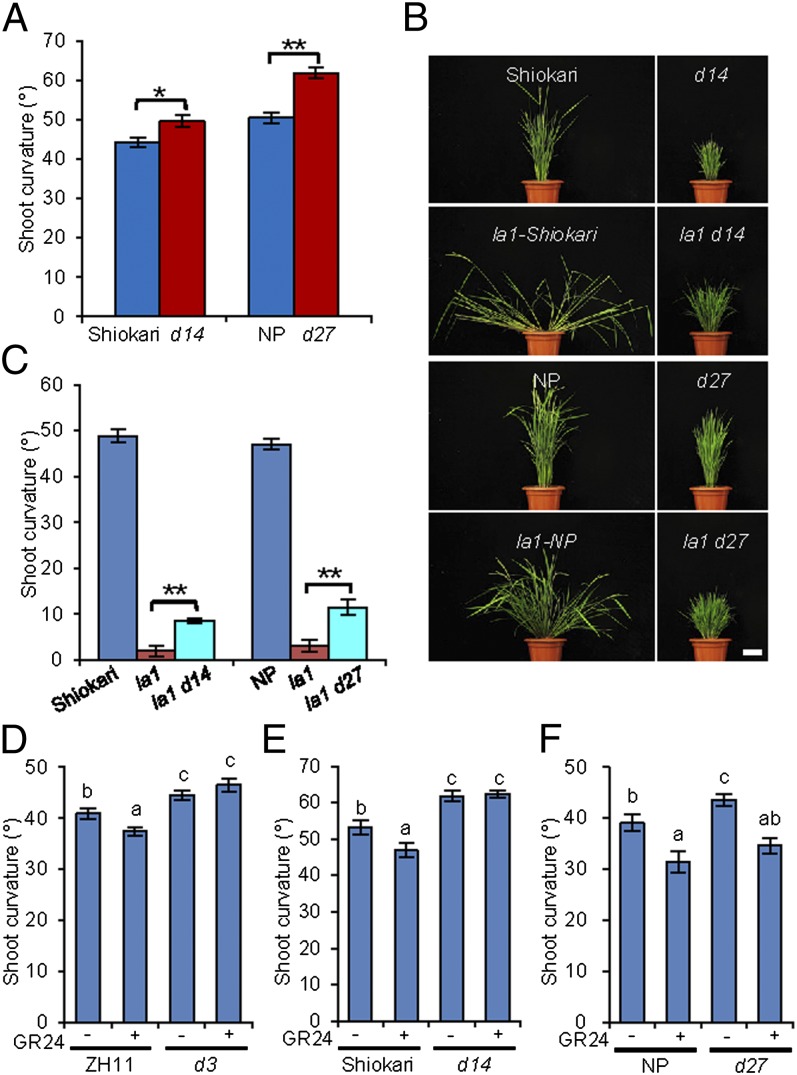
To verify the involvement of SLs in regulating shoot gravitropism, we first examined the shoot gravitropic responses of seedlings of d14 and d27, which were used to represent SL signaling and biosynthetic mutants, respectively. Our results showed that both d14 and d27 seedlings displayed enhanced gravitropic responses compared with the wild-type seedlings (Fig. 2A and Fig. S5 A–D). Next, we constructed double mutants by crossing la1 with d14 and d27, respectively. The tiller angles of the double mutants la1 d14 and la1 d27 were significantly smaller than that of la1 (Fig. 2B). Consistently, the gravitropic responses of la1 d14 and la1 d27 seedlings were enhanced significantly compared with la1 (Fig. 2C and Fig. S5 E–H), indicating that the deficiency in both SL biosynthesis and signaling could rescue shoot gravitropism and the spreading phenotype of the la1 plant.
Fig. 2.
SLs attenuate shoot gravitropic response in rice. (A) Shoot curvature of the wild-type plants [Shiokari and Nipponbare (NP)] and d mutants at the seedling stage after 48 h of gravistimulation. (B) The phenotypes of the la1 d14 and la1 d27 double mutants at the adult stage. (Scale bar: 10 cm.) (C) Shoot curvature of the la1 d14 and la1 d27 double mutants at the seedling stage after 24 h of gravistimulation. In A and C, *P < 0.05, **P < 0.01, Student t test. (D–F) The shoot gravitropic responses of d3 (D), d14 (E), and d27 (F) seedlings upon GR24 treatment. The seedlings were grown under light with or without GR24 treatment for 72 h and then were transferred to darkness and reoriented by 90° upon gravistimulation. Means with different letters are significantly different (P < 0.05; ANOVA). Error bars indicate SEM; n = 10.
To obtain deeper insight into the role of SLs in shoot gravitropism, we analyzed the shoot gravitropic response upon treatment with GR24, a synthetic analog of SLs (31). As shown in Fig. 2 D–F, the gravitropic response of the wild-type seedlings was reduced significantly with GR24 treatment. In contrast, GR24 treatment could not affect the gravitropic response of the d3 or d14 mutant, suggesting that SLs decrease shoot gravitropism in a D3- and D14-dependent manner (Fig. 2 D and E), as is consistent with the role of D3 and D14 in the perception of SLs. However, GR24 could reduce the gravitropic response of the d27 seedlings (Fig. 2F). Taken together, these results suggest that SLs can attenuate shoot gravitropism and thus regulate the rice tiller angle.
SL Regulation of Rice Shoot Gravitropism Is Dependent on Local Auxin Level.
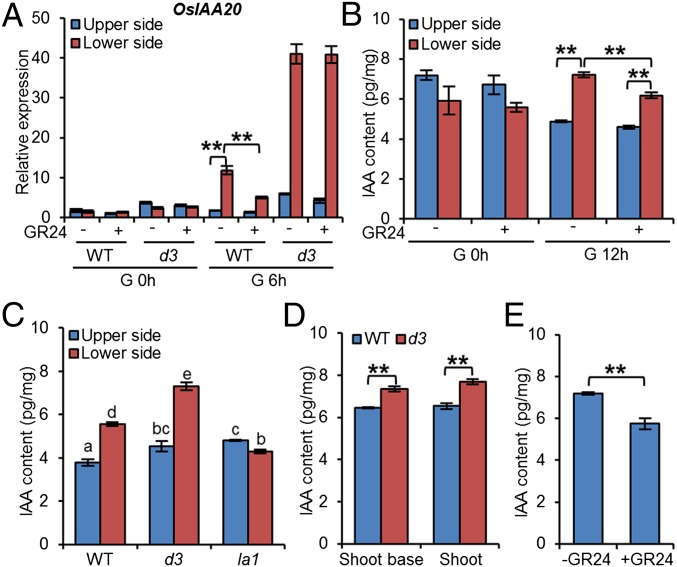
It has been shown that the asymmetric distribution of auxin plays a key role in gravitropism (1–3). To characterize the involvement of auxin in SL-mediated gravitropism, we examined the expression levels of the auxin-responsive marker gene OsIAA20 in the wild-type and d3 seedlings upon GR24 treatment before and after gravistimulation. The results showed that the expression levels of OsIAA20 in the lower side and upper sides were comparable in wild-type and d3 seedlings with or without GR24 treatment before gravistimulation (Fig. 3A and Fig. S6). However, OsIAA20 was expressed asymmetrically after gravity stimulus in the wild-type seedlings, and its expression was reduced significantly in the lower side of the shoot base upon GR24 treatment. OsIAA20 expression was higher in the d3 seedlings than in wild-type seedlings at both the upper and lower sides of the shoot base and did not change with GR24 treatment (Fig. 3A). These results indicate that the SLs may affect gravity-induced auxin redistribution. Furthermore, we measured the indoleacetic acid (IAA) content in the upper and lower sides of the shoot base upon gravity stimulation after GR24 treatment. The results demonstrated that GR24 decreased the IAA level in the lower side more significantly than in the upper side of the shoot base after 12 h of gravistimulation (Fig. 3B), indicating that SLs may regulate asymmetric auxin distribution after gravity stimulus in the shoot base.
Fig. 3.
SLs regulate rice shoot gravitropism by decreasing the local auxin level. (A) Expression levels of OsIAA20 at the lower and upper sides of the shoot base in wild-type and d3 seedlings with or without GR24 treatment upon gravistimulation for 0 and 6 h. Error bars indicate SEM; n = 3. (B) IAA contents at the lower and upper sides of the shoot base with or without GR24 treatment upon gravistimulation for 0 and 12 h. Light-grown 7-d-old seedlings were reoriented by 90° upon gravistimulation. Error bars indicate SEM; n = 4. (C) IAA contents showing an asymmetric auxin distribution between the upper and lower sides of shoot base in the wild-type and d3 and la1 mutant seedlings upon 12 h of gravistimulation. Error bars indicate SEM; n = 3. Means with different letters are significantly different (P < 0.05; ANOVA). (D) The IAA contents in the shoot base and shoot of the wild-type and d3 seedlings. Error bars indicate SEM; n = 4. (E) The IAA contents in the shoot of wild-type seedlings with or without GR24 treatment. Error bars indicate SEM; n = 4. **P < 0.01, Student t test.
Furthermore, we measured the auxin contents of 7-d-old seedlings of the wild type, la1, and d3 mutants after 12 h of gravistimulation. As shown in Fig. 3C, d3 mutants accumulated more auxin in the lower side of the shoot base than did wild-type seedlings. In contrast, in la1 seedlings the auxin level was not elevated at the lower side of shoot base upon gravistimulation. Moreover, we found that in d3 seedlings the IAA level was elevated not only in the shoot base but also in the shoot (Fig. 3D). In contrast, GR24 treatment resulted in decreased IAA levels in the shoots of rice seedlings (Fig. 3E). Collectively, these data demonstrate that SLs may attenuate shoot gravitropism by decreasing the local IAA content via regulating auxin biosynthesis.
SL-Mediated Shoot Gravitropism Is Conserved in Arabidopsis.
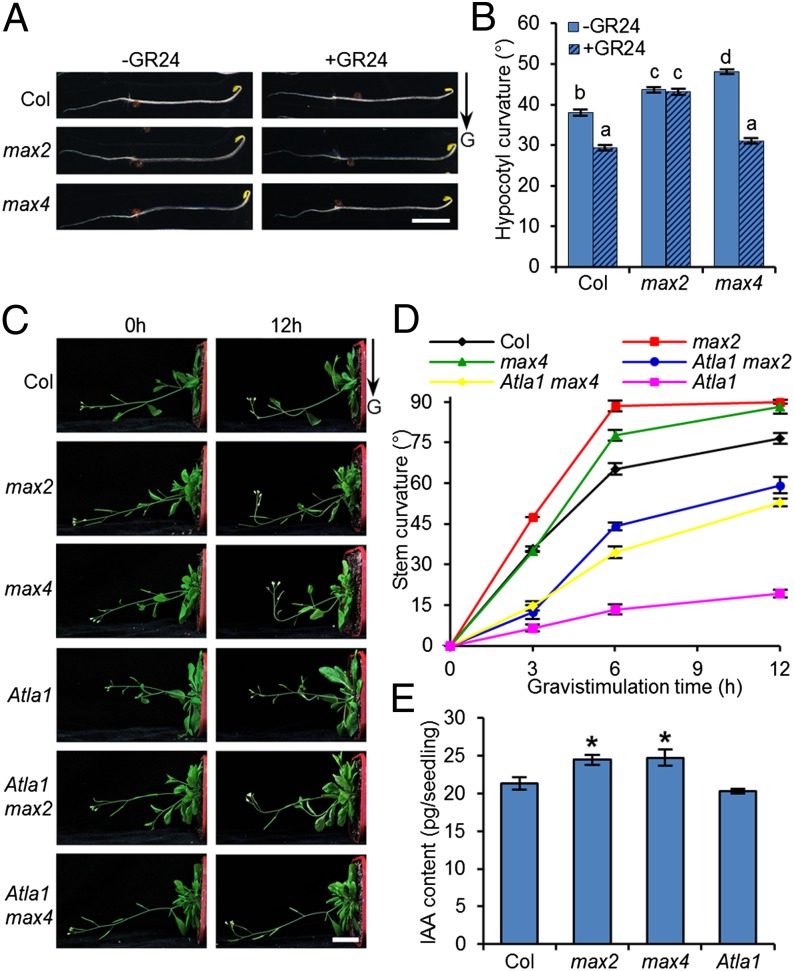
Because the involvement of SLs in shoot branching is conserved in many plant species, we reasoned that the molecular mechanism of SL-mediated shoot gravitropism should be conserved in other species, such as the dicot Arabidopsis. To verify this notion, we examined the gravitropism of Arabidopsis max2 and max4 mutant seedlings. As shown in Fig. 4 A and B, the gravitropic responses of etiolated hypocotyls in max2 and max4 mutants were enhanced significantly as compared with the wild type after 6 h of gravistimulation. Moreover, the hypocotyl gravitropism of the max4 mutant was restored to the level in the wild type upon GR24 treatment, whereas the max2 mutant exhibited consistent enhanced gravitropism upon GR24 treatment (Fig. 4 A and B). Accordingly, the gravitropic responses of max2 and max4 inflorescence stems were enhanced significantly after gravistimulation (Fig. 4 C and D). We then generated the double mutants of Atla1 max2 and Atla1 max4 and compared their gravitropic responses with the responses of Atla1, a gravitropism-defective mutant caused by a mutation in the Arabidopsis LA1 gene (Fig. S7) (32). As shown in Fig. 4 C and D, the gravitropic responses of the double mutants were enhanced compared with those of Atla1. Furthermore, the IAA levels in max2 and max4 seedlings were higher than in the wild type, but the Atla1 IAA level was comparable to that in the wild type (Fig. 4E). These results demonstrated that SLs can regulate shoot gravitropism by auxin biosynthesis in both dicot and monocot plants.
Fig. 4.
Involvement of SLs in shoot gravitropism is conserved in Arabidopsis. (A) Photographs of wild-type (Col-0), max2, and max4 hypocotyls with or without GR24 treatment upon gravistimulation. (Scale bar: 0.5 cm.) (B) Statistic analysis of hypocotyl curvature in 4-d-old dark-grown wild-type, max2, and max4 seedlings with or without GR24 treatment upon 6 h of gravistimulation. Error bars represent SEM; n = 50. Means with different letters indicate a significant difference (P < 0.01; ANOVA). (C) Photographs of inflorescence stems of wild-type, max2, max4, Atla1, Atla1 max2, and Atla1 max4 seedlings upon 12 h of gravistimulation. (Scale bar: 2 cm.) (D) Kinetic analysis of inflorescence stem curvature in wild-type, max2, max4, Atla1, Atla1 max2, and Atla1 max4 seedlings upon gravistimulation. Values represent the mean ± SEM; n = 10. (E) Quantification of IAA levels in 7-d-old light-grown wild-type, max2, max4, and Atla1 seedlings. Error bars indicate SEM; n = 3. *P < 0.05; Student t test.
SL Regulation of Rice Shoot Gravitropism Is Independent of LA1.
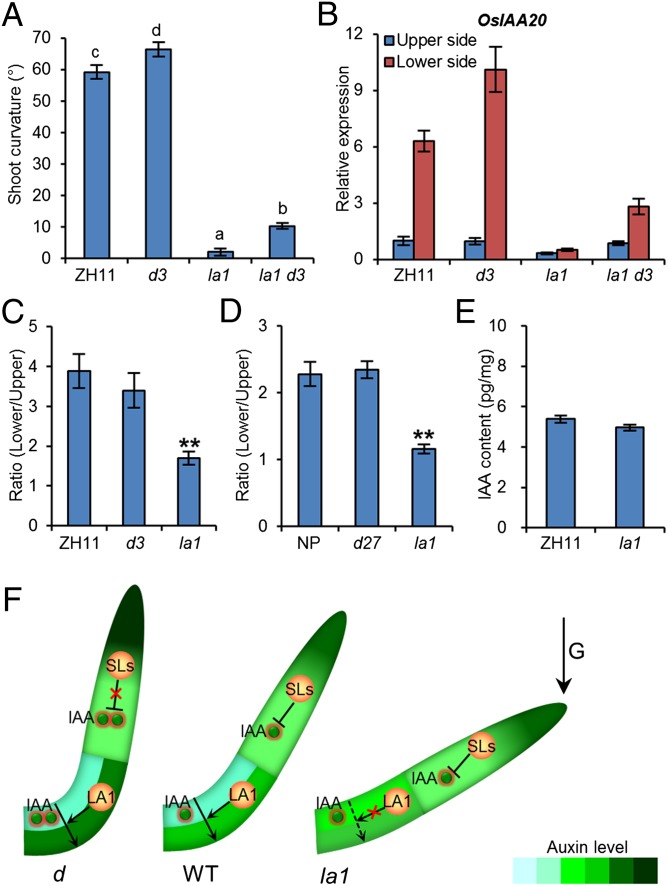
To reveal the relationship between the SLs and LA1 in the regulation of shoot gravitropism, we first examined the D3 expression in la1 and the LA1 expression in d3. The qRT-PCR results showed that D3 expression was comparable in wild-type and la1 seedlings (Fig. S8A), and the expression of LA1 was unchanged in d3 seedlings (Fig. S8B), implying that D3 may regulate shoot gravitropism independent of LA1. Therefore we performed an in-depth analysis of the shoot gravitropism in d3, la1, and la1 d3 seedlings. Upon gravistimulation at the seedling stage, the d3 shoot bent faster than the wild type. The la1 d3 seedling showed a greater gravitropic response than the la1 plant, but this response still was weaker than those of the wild-type and d3 seedlings (Fig. 5A and Fig. S8 C and D). Accordingly, the expression of OsIAA20 at the lower side of the la1 d3 shoot base after 6 h of gravistimulation was significantly higher than in the la1 seedling but was lower than in the wild-type and d3 seedlings (Fig. 5B). These results indicate that D3 and LA1 may have parallel but opposite functions in regulating shoot gravitropism through an asymmetric distribution of auxin.
Fig. 5.
SLs regulate shoot gravitropism in an LA1-independent manner. (A) Comparison of shoot gravitropism in wild-type (ZH11), d3, la1 and la1 d3 seedlings. Light-grown 3-d-old seedlings were transferred to darkness and reoriented by 90° upon gravitropism for 2 d. Error bars indicate SEM; n =10. Means with different letters are significantly different (P < 0.05; ANOVA). (B) Expression levels of OsIAA20 at the lower and upper sides of the shoot base after gravity stimulation for 6 h. Error bars indicate SEM; n = 3. (C and D) Comparison of lateral IAA transport in wild-type, la1, and d3 seedlings (C) and d27 mutants (D). The ratio indicates the radioactivity of the lower side to that of the upper side of coleoptiles upon gravity stimulation. Error bars indicate SEM; n = 10. **P < 0.01; Student t test. (E) Comparison of IAA levels in wild-type and la1 seedlings. Error bars indicate SEM. n = 3. (F) A proposed model of SL- and LA1-dependent shoot gravitropism.
As previously reported, LA1 could regulate the asymmetric auxin distribution through lateral auxin transport (5). In this study, we found that, unlike la1 mutants, mutants defective in the SL biosynthetic and signaling pathways exhibited normal lateral auxin transport (Fig. 5 C and D). We also found that the IAA level was not affected in la1 seedlings (Fig. 5E) but was elevated in the SL-defective mutants (Fig. 3D). These data strongly suggested that SLs may regulate auxin asymmetric distribution and thus shoot gravitropism mainly through auxin biosynthesis, without an obvious effect on lateral auxin transport. Therefore, the SL biosynthetic and signaling pathways very likely are involved in a negative regulation of rice shoot gravitropism and tiller angle in a genetic pathway that is distinct from that of LA1.
Discussion
Rice tillers are specialized branches, and the tiller angle is an important agronomic trait that contributes to plant architecture and grain production (4). In the past years, several genes that determine rice tiller angle have been cloned and characterized (5–9). Some of them, for example LA1 and LPA1, are involved in shoot gravitropism and thus regulate tiller/branch angle in different plant species (5, 9, 32, 33), indicating that shoot gravitropism is the key component dictating the proper positioning of shoot branches. Although the asymmetric distribution of auxin has long been regarded as the main factor that affects shoot gravitropism, the underlying molecular mechanism still remains to be elucidated.
SLs are a group of terpenoid lactones that were known first for their functions in rhizosphere parasitic and symbiotic interactions (31, 34). Recently, SLs have been found to act as plant hormones playing diverse roles in plant growth and development (12). In this study, we revealed a novel role of SLs in regulating shoot gravitropism by screening for sols in rice.
Our results demonstrate that the SLs attenuate shoot gravitropism, which in turn regulates tiller/branch angle. We show that SL biosynthetic and signaling mutants can rescue the la1 phenotype in both rice (Figs. 1A and 2B and Fig. S4) and Arabidopsis (Fig. 4). Consistently, SL-deficient mutants have enhanced shoot gravitropism in both rice seedlings (Fig. 2 A and D and Fig. S5) and Arabidopsis hypocotyls and inflorescence stems (Fig. 4). GR24 treatment, on the other hand, reduces the shoot gravitropic response in the wild-type seedlings and SL biosynthetic mutants (Figs. 2F and 4 A and B), but not in SL signaling mutants (Figs. 2 D and E and 4 A and B). Furthermore, SLs regulate the asymmetric distribution of auxin in the shoot base after gravity stimulus (Fig. 3 A–C). Further evidence showed that SL-deficient mutants have increased IAA content in both the shoot base and whole seedlings (Fig. 3D), but lateral auxin transport is not altered (Fig. 5 C and D). Therefore we concluded that SLs attenuate shoot gravitropism mainly by inhibiting auxin biosynthesis.
As previously reported, PAT is enhanced in both SL-defective and la1 mutants. In the la1 mutant, the enhanced PAT disrupts the lateral auxin transport, leading to an abnormal asymmetric distribution of auxin and shoot gravitropism (5) (Fig. 5 A–E). However, in the SL-defective mutants, the lateral auxin transport is normal (Fig. 5 C and D), although PAT is also enhanced (14, 35). The increased auxin biosynthesis in SL-defective mutants leads to an increased IAA level at the lower side of shoot base upon gravistimulation, by which the shoot gravitropism is enhanced (Fig. 3 C and D). Shoot gravitropism is enhanced significantly in the d/max la1 double mutant compared with the la1 mutant but is attenuated compared with the d/max mutants (Fig. 4 C and D, Fig. 5A, and Fig. S8 C and D), suggesting that the SL biosynthetic and signaling pathways may antagonize LA1 in regulating shoot gravitropism in an LA1-independent manner.
Based on our present results, we proposed a model of rice tiller angle control (Fig. 5F). In the wild-type rice plant, SLs inhibit auxin biosynthesis and maintain a moderate auxin level to generate an asymmetric auxin distribution at the shoot base upon gravistimulation. In SL-defective mutants, SLs seem unable to inhibit the auxin biosynthesis; the increased auxin level at the lower side of the shoot base upon gravistimulation results in an enhanced gravitropic response and a compact plant. Although auxin biosynthesis is normal in the la1 mutant, the PAT is enhanced, and the lateral auxin transport is defective, so that the lower side of shoot base cannot accumulate auxin, eventually resulting in a spreading phenotype. In the la1 d double mutants, the increased auxin level at the lower side of shoot base of d mutants can compensate to some extent for the lack of auxin at the lower side of shoot base of la1 mutants, leading to a compromised tiller angle. As a major hormone in regulating shoot branching/tillering, SLs recently have been shown to interact with auxin by triggering rapid deletion of the auxin efflux protein PIN1 (36). Notably, the tropic response of shoots in pin1 is normal (37). Therefore we proposed that SLs are likely to regulate shoot branching and gravitropism in parallel by auxin transport and biosynthesis.
Taken together, our results uncover a previously unidentified mechanism underlying the involvement of SLs in the control of shoot gravitropism and thus the tiller/branch angle, illustrating a potentially widely conserved strategy by which plants optimize growth and development under diverse environmental conditions.
Materials and Methods
Plant growth, map-based cloning, qRT-PCR, and shoot gravitropic assays were performed as described previously (5, 32), and rice was transformed as in a previous report (38). Free IAA content and lateral auxin transport were measured as previously described (5, 39), with minor modifications. Primers used in this study are listed in Table S1. Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ottoline Leyser and the Arabidopsis Biological Resource Center for providing Arabidopsis seeds. This work was supported by National Natural Science Foundation of China Grants 90817108, 31025004, and 91335204 and Ministry of Science and Technology of the People’s Republic of China Grants 2012AA10A301 and 2013CBA01401.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1411859111/-/DCSupplemental.
References
- 1.Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4(3):103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- 2.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7(6):712–718. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Masson PH, et al. (2009) Signaling in plant gravitropism. Signaling in Plants, eds Baluška F, Mancuso S (Springer, Berlin), pp 209–237. [Google Scholar]
- 4.Wang Y, Li J. The plant architecture of rice (Oryza sativa) Plant Mol Biol. 2005;59(1):75–84. doi: 10.1007/s11103-004-4038-x. [DOI] [PubMed] [Google Scholar]
- 5.Li P, et al. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 2007;17(5):402–410. doi: 10.1038/cr.2007.38. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, et al. TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. 2007;52(5):891–898. doi: 10.1111/j.1365-313X.2007.03284.x. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, et al. Genetic control of rice plant architecture under domestication. Nat Genet. 2008;40(11):1365–1369. doi: 10.1038/ng.247. [DOI] [PubMed] [Google Scholar]
- 8.Tan L, et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat Genet. 2008;40(11):1360–1364. doi: 10.1038/ng.197. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Tang D, Li M, Wang K, Cheng Z. Loose Plant Architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013;161(1):317–329. doi: 10.1104/pp.112.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 11.Umehara M, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455(7210):195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 12.de Saint Germain A, Bonhomme S, Boyer FD, Rameau C. Novel insights into strigolactone distribution and signalling. Curr Opin Plant Biol. 2013;16(5):583–589. doi: 10.1016/j.pbi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Alder A, et al. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science. 2012;335(6074):1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, et al. DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21(5):1512–1525. doi: 10.1105/tpc.109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seto Y, et al. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc Natl Acad Sci USA. 2014;111(4):1640–1645. doi: 10.1073/pnas.1314805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso C, et al. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc Natl Acad Sci USA. 2014;111(6):2379–2384. doi: 10.1073/pnas.1317360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa S, et al. Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 2005;46(1):79–86. doi: 10.1093/pcp/pci022. [DOI] [PubMed] [Google Scholar]
- 18.Stirnberg P, Furner IJ, Leyser O. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50(1):80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson X, et al. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006;142(3):1014–1026. doi: 10.1104/pp.106.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arite T, et al. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50(8):1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z, et al. Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Mol Biol. 2009;71(3):265–276. doi: 10.1007/s11103-009-9522-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, et al. Identification and characterization of HTD2: A novel gene negatively regulating tiller bud outgrowth in rice. Planta. 2009;230(4):649–658. doi: 10.1007/s00425-009-0975-6. [DOI] [PubMed] [Google Scholar]
- 23.Hamiaux C, et al. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr Biol. 2012;22(21):2032–2036. doi: 10.1016/j.cub.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LH, et al. Crystal structures of two phytohormone signal-transducing α/β hydrolases: Karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 2013;23(3):436–439. doi: 10.1038/cr.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura H, et al. Molecular mechanism of strigolactone perception by DWARF14. Nat Commun. 2013;4:2613. doi: 10.1038/ncomms3613. [DOI] [PubMed] [Google Scholar]
- 26.Zhou F, et al. D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504(7480):406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L, et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504(7480):401–405. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer PB, Koltai H, Beveridge CA. Diverse roles of strigolactones in plant development. Mol Plant. 2013;6(1):18–28. doi: 10.1093/mp/sss130. [DOI] [PubMed] [Google Scholar]
- 29.Ha CV, et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc Natl Acad Sci USA. 2014;111(2):851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challis RJ, Hepworth J, Mouchel C, Waites R, Leyser O. A role for MORE AXILLARY GROWTH1 (MAX1) in evolutionary diversity in strigolactone signaling upstream of MAX2. Plant Physiol. 2013;161(4):1885–1902. doi: 10.1104/pp.112.211383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara T, Spalding EP, Iino M. AtLAZY1 is a signaling component required for gravitropism of the Arabidopsis thaliana inflorescence. Plant J. 2013;74(2):267–279. doi: 10.1111/tpj.12118. [DOI] [PubMed] [Google Scholar]
- 33.Dong Z, et al. Maize LAZY1 mediates shoot gravitropism and inflorescence development through regulating auxin transport, auxin signaling, and light response. Plant Physiol. 2013;163(3):1306–1322. doi: 10.1104/pp.113.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook CE, Whichard LP, Turner B, Wall ME, Egley GH. Germination of witchweed (Striga Lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;154(3753):1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 35.Bennett T, et al. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006;16(6):553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara N, Taylor C, Leyser O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013;11(1):e1001474. doi: 10.1371/journal.pbio.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3(7):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6(2):271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 39.Fu J, Chu J, Sun X, Wang J, Yan C. Simple, rapid, and simultaneous assay of multiple carboxyl containing phytohormones in wounded tomatoes by UPLC-MS/MS using single SPE purification and isotope dilution. Anal Sci. 2012;28(11):1081–1087. doi: 10.2116/analsci.28.1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.