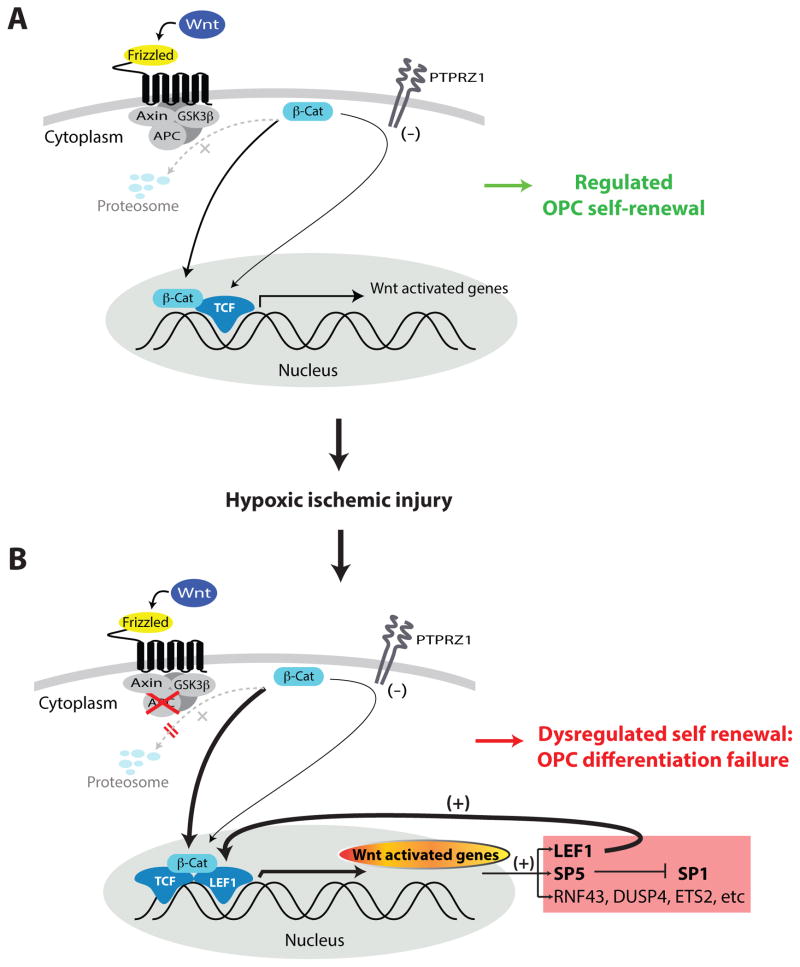
Fig. 1. Neonatal hypoxic-ischemic injury can yield dysregulated Wnt/βcatenin signaling and sustained maturational arrest by oligodendrocyte progenitor cells.
A, The homeostatic self-renewal of OPCs in healthy forebrain white matter is modulated through the regulated nuclear translocation of βcatenin, its binding therein to TCF-family transcription factors, and the resultant transcription of TCF-regulated target genes. Extracellular signals that modulate this process include the Wnt proteins in their binding to Frizzled-family receptors, the activation of which down-regulates GSK3β kinase activity, which would otherwise target cytoplasmic catenin for proteosomal degradation. Concurrent self-renewal signals also include the receptor tyrosine phosphatase β/ζ (PTPRZ1), which acts in concert with GSK3β to tonically suppress catenin availability (ref. 9).
B, Following hypoxic-ischemic injury to white matter OPCs, βcatenin-mediated TCF-dependent transcription increases, while expression of its negative regulator APC falls. These cells mimic the dysregulated Wnt/βcatenin-signaling noted in APC deficient colon cancers, with which they share up-regulation of a discrete set of Wnt-modulated transcriptional target genes. This tonically up-regulated gene set includes the LEF1 transcription factor, which drives its own expression in a feed-forward manner to sustain high levels of Wnt-associated gene expression. These transcripts also include the SP/KLF-family transcription factor SP5, which serves to sustain self-renewal competence, and whose knock-down yields precocious differentiation. The feed-forward nature of this dysregulated LEF over-expression appears to render OPCs refractory to terminal oligodendrocytic differentiation, instead favoring persistence in the progenitor state. However, unlike the neoplastic expansion seen in APC-mutant or deficient colon carcinomas, the sustained Wnt/βcatenin signal activation and diminished APC expression seen in OPCs following hypoxic-ischemic injury is associated with maturational arrest, and not with facilitated cell division or tumorigenesis, thus highlighting the effects of phenotype and local tissue context on the cellular outcomes of dysregulated Wnt signaling.