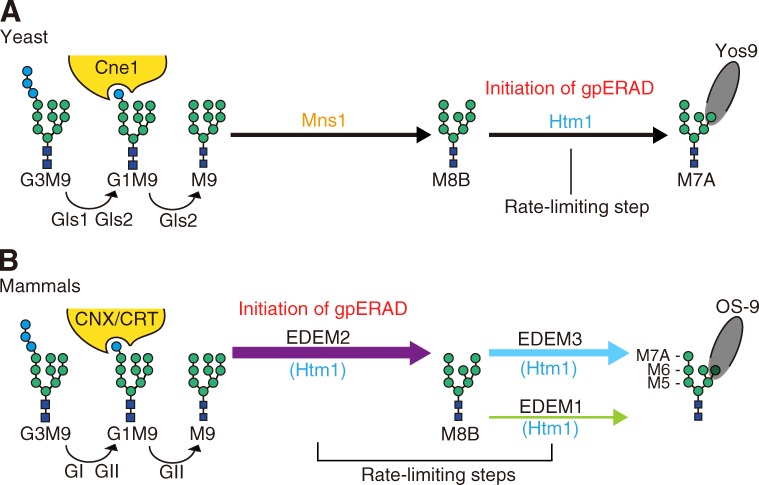
Figure 5.
Models of yeast and mammalian gpERAD. (A) In yeast, high-mannose-type oligosaccharide attached to asparagine (Glc3Man9GlcNAc2, G3M9) is first trimmed to M9 by glucosidases Gls1 and Gls2. M9 is trimmed to M8B by Mns1 and M8B is trimmed to M7A by Htm1. G1M9 is recognized by lectin chaperone Cne1 for folding, whereas M7A exposing α1,6-mannose is recognized by lectin Yos9 for subsequent degradation. The rate-limiting step in yeast gpERAD is the trimming from M8B to M7A. Refer to reviews by Molinari (2007) and Hosokawa et al. (2010a). (B) In mammals, G3M9 is trimmed to M9 by glucosidase I (GI) and glucosidase II (GII), homologues of yeast Gls1 and Gls2, respectively. G1M9 is recognized by lectin chaperones calnexin (CNX) and calreticulin (CRT), homologues of yeast Cne1, for folding. As our current results unambiguously show that M9 is mainly trimmed to M8B by EDEM2 and that M8B is trimmed by EDEM1 and EDEM3 to Man7-5GlcNAc2, which are recognized by lectin OS-9, a homologue of yeast Yos9, for degradation, mammalian gpERAD has two rate-limiting steps.