Table 5.
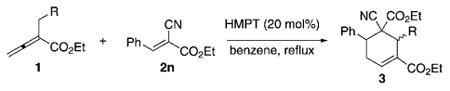
Survey of allenoates 1 for [4+2] annulations with benzylidenecyanoacetate 2n.[a]
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | R | Product | Yield [%][b] | cis/trans[c] |
| 1 | Ph (1b) | 3w | 81 | 88:12 |
| 2 | 4-ClC6H4 (1c) | 3x | 74 | 89:11 |
| 3 | CO2Et (1d) | 3y | 78 | 77:23 |
Reaction conditions: A solution of the allenoate 1 (1.4–3.0 mmol) in benzene (10 mL) was added to a solution of the olefin 2n (1 mmol) and HMPT (20 mol%) in benzene (5 mL) under reflux over 4 h and then the mixture was further heated under reflux.
Yield of isolated product.
Determined through analysis of the 1H NMR spectrum of the crude reaction mixture and by comparison with the spectrum of cis-ethyl 5,5-dicyano-4,6-diphenylcyclohex-1-enecarboxylate (for which a single-crystal X-ray structure was obtained). See Ref. [8].
