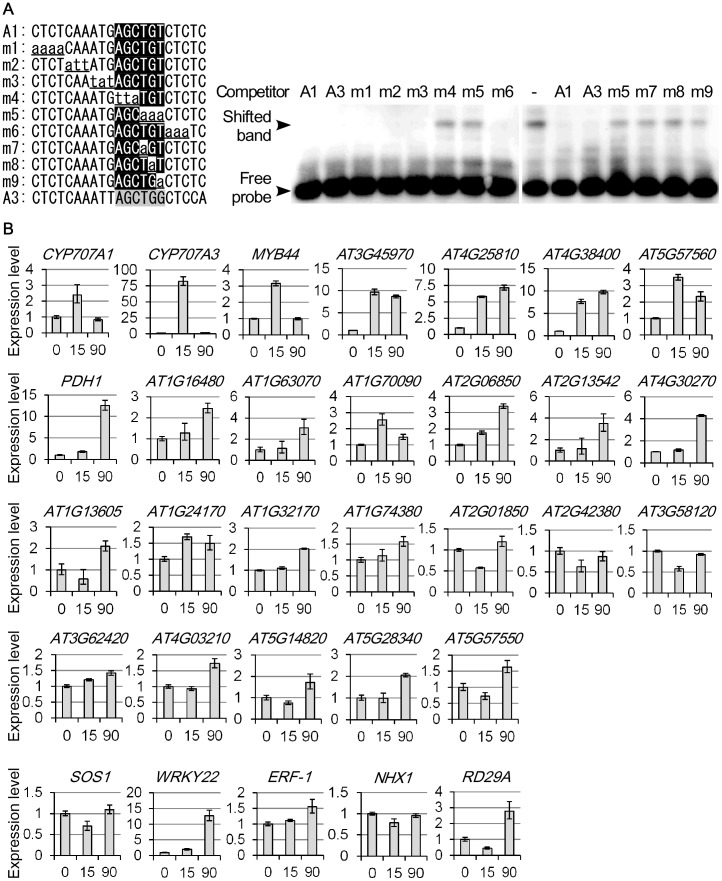
Figure 2. Interaction between VIP1 and AGCTGT/G and its relevance to hypo-osmolarity- and/or submergence-induced gene expression.
(A) VIP1 binds AGCTGT/G. The gel shift assay was performed using purified GST-fused VIP1ΔN164 and a DIG-labeled CYP707A1 promoter fragment as the probe. The 21-bp CYP707A1/3 promoter fragments (A1 for the CYP707A1 promoter and A3 for the CYP707A3 promoter) and point-mutated versions of A1 (m1-9) were used as competitors. In the left panel, sequences mutated from A1 in m1-9 are in lower case and underlined. AGCTGT and AGCTGG in the competitors are indicated by black and gray boxes, respectively. “Competitor -” indicates the absence of the competitors in the reaction mixture. The results of A1 and A3 are shown in both the middle and the right panels for controls. The experiments were performed three times, and a representative result is shown. (B) Quantitative RT-PCR analyses of expression of the genes with promoters containing at least one copy of AGCTGT/G (see Dataset S2 for the exact copy numbers of AGCTGT/G in the promoters of these genes) under a hypo-osmotic condition. Two-week-old wild-type seedlings were submerged in 20 mM Tris-HCl for 0, 15 and 90 minutes, and sampled for RNA extraction and cDNA synthesis. Numbers on x-axes indicate the time points of sampling. Genes up-regulated (>2 fold) either 15 or 90 minutes after the hypo-osmotic treatment are shown in the first two rows. Genes that were not up-regulated by the hypo-osmotic treatment are shown in the third and fourth rows. Genes in the bottom row (SOS1, WRKY22, ERF-1, NHX1 and RD29A) do not have AGCTGT/G in the promoters and are shown as controls. Relative expression levels were calculated by the comparative cycle threshold (CT) method using UBQ5 as an internal control gene. Data are means of three biological replicates. Error bars indicate SD.