The histological diversity of breast carcinomas has relevant prognostic implications. Special histologies can be very rare, and the majority of information on outcome and treatments derives from small series and case reports. As a consequence, clear recommendations about clinical management are still lacking. This review summarizes current knowledge about rare breast cancer histologies.
Keywords: Mucinous carcinoma, Tubular carcinoma, Medullary carcinoma, Metaplastic carcinoma, Pleomorphic lobular cancer, Cribriform carcinoma
Abstract
Breast cancer encompasses a collection of different diseases characterized by different biological and pathological features, clinical presentation, response to treatments, clinical behavior, and outcome. On the basis of cell morphology, growth, and architecture patterns, breast cancer can be classified in up to 21 distinct histological types. Breast cancer special types, including the classic lobular invasive carcinoma, represent 25% of all breast cancers. The histological diversity of breast carcinomas has relevant prognostic implications. Indeed, the rare breast cancer group includes subtypes with very different prognoses, ranging from the tubular carcinoma, associated with an indolent clinical course, to metaplastic cancer, whose outcome is generally unfavorable. New approaches based on gene expression profiling allow the identification of molecularly defined breast cancer classes, with distinct biological features and clinical behavior. In clinical practice, immunohistochemical classification based on the expression of human epidermal growth factor receptor 2 and Ki67 is applied as a surrogate of the intrinsic molecular subtypes. However, the identification of intrinsic molecular subtypes were almost completely limited to the study of ductal invasive breast cancer. Moreover, some good-prognosis triple-negative histotypes, on the basis of gene expression profiling, can be classified among the poor-prognosis group. Therefore, histopathological classification remains a crucial component of breast cancer diagnosis. Special histologies can be very rare, and the majority of information on outcome and treatments derives from small series and case reports. As a consequence, clear recommendations about clinical management are still lacking. In this review, we summarize current knowledge about rare breast cancer histologies.
Abstract
摘要
乳腺癌包括一系列不同的疾病,这些疾病的特点为具有不同的生物学和病理学特征、临床表现、对治疗的反应、临床行为以及预后。根据细胞形态学、生长和结构模式,乳腺癌最多可以分成 21 种截然不同的组织学类型。乳腺癌特殊类型(包括典型浸润性小叶癌)占所有乳腺癌的 25%。乳腺癌的组织学多样性有相关的预后启示。实际上,罕见的乳腺癌组包括预后截然不同的亚型,从小管癌(具有惰性的临床过程)到化生性癌(预后通常不良)。基于基因表达分析的新方法可以识别根据分子确定的乳腺癌类型,这些类型具有截然不同的生物学特征和临床行为。在临床实践中,基于人表皮生长因子受体 2 和 Ki67 表达的免疫组织化学分类代替固有分子亚型得以应用。然而,固有分子亚型的识别几乎完全局限于浸润性乳腺导管癌的研究。而且,根据基因表达分析,一些预后良好的三阴性组织分型可能归入预后不佳组。因此,组织病理学分类依然是乳腺癌诊断的关键环节。特殊组织学类型非常罕见,有关结果和治疗的大部分信息源于较小的系列和病例报告。因此,有关临床管理的明确建议仍然缺乏。本综述总结了当前有关罕见乳腺癌组织学类型的知识。 (The Oncologist) 2014;19:805–813
Implications for Practice:
Breast cancer special histologies encompass a panel of various entities with peculiar clinical behaviors. The rarity of such histotypes has jeopardized an extensive clinical evaluation. A review of current available data may help physicians in their clinical practice; however, the development of clear clinical recommendations is not possible. Decisions on the management of patients with rare breast cancer histologies should derive from careful case-by-case multidisciplinary evaluations.
Introduction
Breast cancer (BC) heterogeneity can be found at different levels, from the classic histopathological characterization to the more modern molecular classification. Indeed, BC encompasses a collection of different diseases characterized by different biological and pathological features, clinical presentation, response to treatments, clinical behavior, and outcome. Pathologists have been aware of the histological diversity of breast carcinomas for a long time. According to the World Health Organization (WHO) classification, BC can be classified in up to 21 distinct histological types on the basis of cell morphology, growth, and architecture patterns (Fig. 1) [1]. The most common histological type is invasive ductal breast carcinoma of no special type (NST), which includes those cancers without peculiar features to merit classification in one special type. BC special types, including the classic lobular invasive carcinoma, represent 25% of all breast cancers [2]. The histopathological classification has a prognostic value [3]. Two extreme examples are the tubular carcinoma, associated with an excellent prognosis, and metaplastic cancer, whose outcome is generally unfavorable [4, 5].
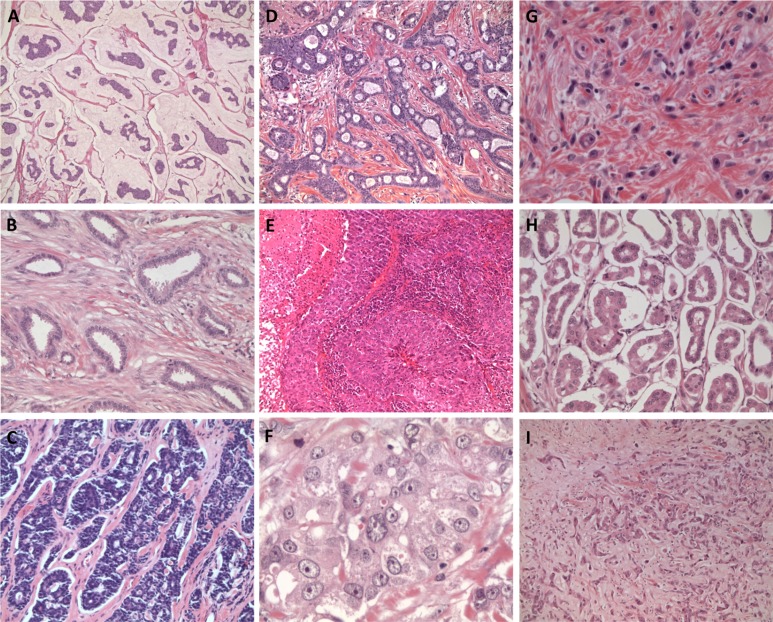
Figure 1.
Histological special types of breast cancer. (A): Mucinous carcinoma. (B): Tubular carcinoma. (C): Adenoid cystic carcinoma. (D): Cribriform carcinoma. (E): Medullary carcinoma. (F): Apocrine carcinoma. (G): Lobular pleomorphic carcinoma. (H): Micropapillary carcinoma. (I): Metaplastic spindle cell carcinoma.
Nowadays, tumor biology is the main determinant of breast cancer treatment. On the basis of immunohistochemistry (IHC), BC consists of at least three main groups: hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-positive, and triple-negative disease (HR- and HER2-negative).
Endocrine manipulation is the cornerstone of therapy for HR-positive tumors; anti-HER2 agents combined with chemotherapy or endocrine therapy are the standard treatment for tumors overexpressing HER2. Chemotherapy represents the only approach for the treatment of triple-negative breast cancers, because no targeted therapy is available so far.
More recently, the further segmentation of BC into intrinsic molecular subtypes based on different gene expression profiling has allowed the identification of at least four different subgroups with different prognosis and sensitivity to treatment: luminal, normal-like, HER2-enriched, and basal-like [6]. HR-positive tumors mainly cluster within the luminal subtype, which can be further divided into two entities: Luminal A and Luminal B, with the latter mainly represented by HR+/HER2+ or HR+ tumors with high Ki67 levels [7]. HER2+/HR− tumors and triple-negative tumors mainly cluster within the HER2-enriched and basal-like subgroups, respectively. Despite the fact that BC classification based on routine pathologic parameters such as HR, HER2, and Ki67 does not completely recapitulate the gene expression profiling-based molecular classification, it is nowadays applied in clinical practice as a surrogate for the intrinsic molecular subtype definition [8, 9].
However, this approach still presents some pitfalls. First, intrinsic molecular subtypes were identified on the basis of gene expression profiles. However, in recent years, we have observed an exponential development of high-throughput technologies and their application in cancer research. The introduction of such techniques has indeed revealed deeper levels of tumor heterogeneity. As an illustration, the integration of gene expression and gene copy number data recently led to the identification of at least 10 different clusters of primary BC, with different prognoses [10]. Thus, molecular classification as a field may be considered a work in progress. Second, the main studies that led to the identification of intrinsic molecular subtypes were almost completely limited to ductal invasive breast cancers of no special type and did not take uncommon histologies into account. Thus, molecular classification is more a description of the heterogeneity of invasive ductal carcinomas rather than an exhaustive representation of the entire BC landscape. From this perspective, a relevant study has been conducted by Weigelt et al. [11]. A panel of BC of special types (including invasive lobular, mucinous, neuroendocrine, apocrine, invasive ductal with osteoclastic giant cells, micropapillary, adenoid cystic, metaplastic, and medullary carcinoma) was analyzed by gene expression profiling. The first relevant finding was that by hierarchical analysis, some of the special types (like micropapillary carcinomas) constituted a distinct and separate cluster of tumors, suggesting a high homogeneity at the molecular level. Moreover, when classified according to the intrinsic molecular classification, each BC special type (except from apocrine carcinoma) fell in one molecular subtype only. On the other hand, within invasive ductal cancers of no special type and classic invasive lobular cancers, all molecular subtypes were represented, suggesting that each rare histotype is more homogeneous at the transcriptome level than more common entities. This was also confirmed at the genomic level, with the demonstration of similar patterns of gene copy number variations for samples of the same rare histology [12]. In addition, some good-prognosis triple-negative histotypes are classified as basal-like cancers based on gene expression profiling, which is the intrinsic molecular subtype with the worst prognosis [11]. As a consequence, treatment decisions based on molecular features not taking into account histological aspects may erroneously recommend unnecessary aggressive therapies for good-prognosis patients.
The main studies that led to the identification of intrinsic molecular subtypes were almost completely limited to ductal invasive breast cancers of no special type and did not take uncommon histologies into account. Thus, molecular classification is more a description of the heterogeneity of invasive ductal carcinomas rather than an exhaustive representation of the entire BC landscape.
In this review, we examine current knowledge about rare BC histologies from a clinical point of view. In addition, when applicable, evidence about specific genomic drivers of rare BC entities will be also described.
Clinicopathological Features of Rare BC Subtypes
Good-Prognosis Group
Typically Hormone Receptor Positive
Pure mucinous carcinoma is characterized by the production of abundant extracellular and/or intracellular mucin. The definition of pure mucinous carcinoma consists of nests of tumor cells floating in lakes of mucin, whereas the mixed form also contains common infiltrating ductal carcinoma NST [13–15]. A precisely defined threshold in the percentage of mucinous component for the distinction between pure and mixed mucinous carcinoma is not well established. However, pure mucinous carcinomas are generally defined as containing more than 90% of mucin, and mixed mucinous carcinomas are those containing 50%–90% of mucin [16]. The presence of less than 50% of mucin identifies ductal carcinoma with a mucinous component. Pure mucinous carcinoma accounts for 1%–4% of all breast cancers, and it is generally diagnosed at older ages. In a retrospective series of 11,400 cases of pure mucinous carcinoma, the median age at diagnosis was 71 years versus 61 years observed in patients with infiltrative ductal carcinomas [17].
The most common mammographic appearance of pure mucinous carcinoma is a low-density mass lesion with well-defined margins. On the sonographic examination, these lesions present isoechogenic echo texture relative to subcutaneous fat [18].
These lesions are mostly well-differentiated HR+ and HER2−. The axillary lymph nodes are rarely involved. These features account for the favorable prognosis of this BC subtype, with a 5-year BC-specific survival rate of 94% compared with 82% of the infiltrating ductal carcinoma counterpart. The overall more favorable outcome is maintained over very prolonged follow-up. The 10-, 15-, and 20-year survival rates were 89%, 85%, and 81%, respectively, for pure mucinous cases compared with 72%, 66%, and 62%, respectively, for infiltrating ductal carcinoma cases [17]. In a large cohort including more than 1,200 cases, the 5-year overall survival (OS) of patients with mucinous carcinoma was not different from the OS of females from the age-matched general population [19].
Tubular carcinoma accounts for less than 2% of invasive breast cancers [20]. For the definition of pure tubular carcinoma, at least 90% of the tumor should present tubular architecture, composed of small round or oval tubules of single layer of epithelial cells that abut directly onto the adjacent desmoplastic stroma. These lesions are usually detected by screening mammography. The radiological appearance is a small spiculated mass that mimics infiltrating ductal carcinoma or radial scars [21].
Tubular carcinomas are nearly always hormone receptor-positive and well-differentiated, with low proliferation. HER2 is generally neither overexpressed nor amplified [22]. When compared with invasive carcinoma of no special type, tubular carcinoma is more likely to be diagnosed at older age and be smaller in size. Nodal involvement is reported in the range of 4%–17%. The prognosis of patients with tubular carcinoma is very good. The largest series so far encompasses 444 patients with tubular carcinoma [19]. The 5-year disease-free survival (DFS) and OS were 94% and 88%, respectively, for tubular carcinoma versus 80% and 77%, respectively, for carcinoma “not otherwise specified”. More interestingly, the 5-year OS was similar to age-matched female sets from the general population.
Invasive cribriform carcinomas (ICCs) account for 0.1%–0.6% of breast cancers and are characterized by an invasive component showing a predominantly cribriform pattern [23]. The median age at diagnosis is 54–63 years [23, 24]. These tumors are subdivided into pure and mixed. In pure ICCs, the growth pattern is cribriform in more than 90% of the lesion. ICCs with a cribriform pattern and a limited extent of tubular invasive elements (less than 50%) are included in pure subtype. Mixed ICCs also contain areas of less-well-differentiated invasive carcinoma [25]. ICCs are generally estrogen receptor-positive (ER+), low grade, and low proliferating. Axillary lymph-nodal metastases are reported in approximately 10% of the cases [26]. ICC has an excellent prognosis in its pure form, whereas more caution is needed for the mixed variants.
Neuroendocrine carcinomas of the breast are defined by the diffuse expression of neuroendocrine markers along with the presence of morphologic neuroendocrine features. The WHO classification describes three main histologic types: neuroendocrine tumor, well-differentiated neuroendocrine carcinoma, and poorly differentiated/small cell and invasive breast carcinoma with neuroendocrine differentiation [2, 27]. The prevalence of the well-differentiated neuroendocrine tumor is up to 0.5% of breast cancers [28]. With IHC, synaptophysin or chromogranin must be expressed in >50% of the cells. These tumors are generally low-grade ER+, and PgR+, and HER2−, with favorable prognosis [29]. The high-grade small-cell variant is described among the poor prognostic group.
Typically Hormone Receptor Negative
Medullary carcinoma represents less than 2% of breast carcinoma and occurs more frequently in younger women [1]. It is composed of poorly differentiated cells, with large vesicular nuclei and prominent nucleoli, arranged in syncytial architecture with circumscribed margins. The distinctive feature is a prominent lymphocytic infiltrate, both within the tumor and at the periphery [30, 31]. These features must be present in the entire tumor for the diagnosis of classical medullary carcinoma. Cases that do not fulfill all these criteria are defined as atypical medullary carcinoma or carcinoma with medullary features. The mammographic appearance is a mass with circumscribed margins; with sonography, medullary carcinoma generally appears as homogeneous or inhomogeneous hypoechoic mass with well-defined margins [32]. Magnetic resonance appearance is often indicative of a benign lesion [33].
Most of medullary cancer presents with triple-negative assets at immunohistochemistry, with cytokeratin 5/6 positivity [34, 35]. The majority of medullary carcinomas are aneuploid and highly proliferative. Despite these unfavorable histologic features, the prognosis of patients with medullary carcinoma is generally good. The medullary histotype is more common in the case of BRCA1 mutations. According to a study including more than 3,600 cases from the Breast Cancer Family Registry consortium, the prevalence of medullary histotype in BRCA1-associated tumors exceeds 16% [36].
The incidence of nodal involvement is lower than other carcinomas of the breast. In a case series including 46 cases, the 10-year-distant relapse-free survival reached 95% [34].
Adenoid cystic carcinoma of the breast is very rare, accounting for less than 1% of breast cancers. Adenoid cystic carcinoma predominantly affects postmenopausal women, with a median age at diagnosis of 60 years [37]. Morphologically, these tumors are similar to adenoid cystic carcinomas arising in the salivary glands, showing epithelial cells and myoepithelial cells arranged in tubular and cribriform architecture. The mitotic activity is generally low.
Mammographically, adenoid cystic carcinoma presents as either irregular masses or growing asymmetric densities, with minimal vascularity on color Doppler imaging [38]. Lymph-nodal involvement is reported in 0%–8% of the cases in the largest published series [39]. With IHC, these tumors are generally HR− and HER2− [40]. The prognosis is generally good, with 10-year OS exceeding 90% [41, 42]. Metastases are rare and generally spread many years after diagnosis, independently from axillary nodal involvement at initial diagnosis. The lung is one of the most frequent sites of distant recurrence. However, even in the presence of local or distant recurrence, patients have a prolonged and indolent clinical course [37].
This extremely rare tumor is one of the salivary gland-like tumors of the breast. At imaging, it appears as a well-circumscribed mass. This tumor generally lacks the expression of hormone receptors and HER2. A positive stain for epithelial membrane antigen and S100 protein is frequently reported [43]. This tumor is generally described as having a good prognosis. However, a note of caution is required, taking into account the paucity of available data.
Also known as juvenile carcinoma, secretory breast carcinoma is extremely rare, representing 0.1%–0.2% of all breast cancers. It accounts for most of the breast cancers diagnosed in childhood. The distinctive pathological characteristics are intracellular or extracellular secretion and granular eosinophilic material [44]. The median age at diagnosis is 25–40 years [45, 46]. The ultrasonography (US) appearance generally resembles a benign lesion [47].
Secretory breast cancers are usually ER-, PgR-, and HER2-negative and have low Ki67 expression. These tumors generally harbor the t(12;15)ETV6-NTRK3 translocation [48]. The ETV-NTRK fusion results in the expression of a functional tyrosine kinases with potent transforming activity. Secretory carcinoma is associated with a good prognosis. Few metastatic cases are described; recurrences generally occurred after very prolonged disease-free intervals (12–20 years) [49].
Intermediate Prognosis
Apocrine Carcinoma
The apocrine epithelium is a normal constituent of apocrine glands, consisting of cells with eosinophilic cytoplasm and a large nucleus located near the base of the cell [50]. Apocrine phenotype is observed in a spectrum of breast epithelial lesions, ranging from benign metaplasia to apocrine carcinoma. The incidence of apocrine carcinoma is reported in the range of 0.3%–4% of all cases, mostly because of the lack of uniform criteria for its diagnosis. The more stringent definition considers as apocrine carcinoma only those neoplasms composed entirely or predominantly of apocrine-type epithelium [51]. Pure apocrine carcinomas are generally ER− and progesteron receptor (PgR)−, and androgen receptor (AR) positive [52]. HER2 is overexpressed in up to 54% of the cases [53]. Mammographic presentation do not differ from that of ductal carcinoma. Conflicting data are available on the outcome of invasive apocrine carcinoma. Some studies report that the prognosis of apocrine carcinoma is the same with other types of breast carcinoma, with the outcome depending on grade, HER2, and hormone receptor expression. A recently published retrospective analysis has included more than 6,800 cases of infiltrating ductal carcinoma NST and 72 cases of apocrine carcinoma. In this study, apocrine carcinomas were subdivided in pure apocrine carcinoma (ER−, PgR−, AR+) and apocrine-like carcinoma (ER or PgR+, AR−) [54]. The diagnosis of pure apocrine carcinoma was independently correlated with a worse DFS, whereas infiltrating ductal carcinoma and apocrine-like BC showed similar outcomes. However, when considering only triple-negative breast cancers, the prognosis of the apocrine histotype was intermediate between the histotypes with the best outcome (like medullary carcinomas) and the ones determining the worst outcome (like metaplastic carcinomas) [55].
Poor-Prognosis Group
Pleomorphic Lobular Carcinoma
Pleomorphic lobular carcinoma is a very rare and aggressive variant of lobular carcinoma, accounting for <1% of all BCs [56, 57]. Pleomorphic lobular carcinoma is characterized by a diffuse spreading pattern arranged in single lines similar to classic invasive lobular cancer. However, the pleomorphic variant is composed of cells with more evident nuclear atypia and pleomorphism [58].
Pleomorphic lobular carcinoma generally affects postmenopausal women [59]. Similarly to the classic variant, pleomorphic lobular carcinoma generally presents with hormone receptor expression and negative staining for E-cadherin. These tumors, however, show a more aggressive nature, evidenced by higher-grade cytological features, the presence of lymphovascular invasion, and a more advanced stage at presentation. HER2 is overexpressed in up to 30% of the cases. Pleomorphic lobular cancer shares the same radiological challenges of the classic lobular counterpart [60]. The more aggressive biological features account for the overall poorer prognosis as compared with classic lobular carcinoma, being the outcome of pleomorphic lobular cancer patients similar to high-grade, invasive ductal carcinoma patients [51].
Invasive Micropapillary Carcinoma
Invasive micropapillary carcinoma is characterized by extensive lymphatic vessel invasion [1, 61]. This feature accounts for the high propensity for nodal metastases, reported in up to 70% of the cases, as well as for the high incidence of early recurrences in skin and in the chest wall [62, 63]. Median age at diagnosis is 52.5 years. With mammography, invasive micropapillary carcinoma appears as high-density mass, generally with spiculated margins [64]. The expression of HR is reported in approximately two-thirds of the cases. A positive HER2 status is described in up to 50% of the cases. This subtype is associated with a poor prognosis. In a study including 98 cases of invasive micropapillary carcinoma, the 10-year OS was 48% [65].
Metaplastic Carcinoma
Metaplastic breast cancers are a heterogeneous group of tumors characterized by differentiation of the neoplastic epithelium into squamous or mesenchymal phenotype. Recently the WHO Working Group proposed this descriptive classification including squamous cell carcinoma, spindle cell carcinoma, metaplastic carcinoma with mesenchymal differentiation, and mixed metaplastic carcinoma. The metaplastic carcinoma group also includes low grade adenosquamous carcinomas and fibromatosis-like metaplastic carcinomas that are associated with more favorable prognosis [1]. The incidence of metaplastic breast carcinoma is in the range of 0.2%–0.6%, and the median age at presentation is 47–61 years [66–68].
A relatively high proportion of metaplastic carcinomas are diagnosed in Hispanic or black women [1, 4]. With mammography, metaplastic carcinoma usually appears as a round, lobular, oval lesion with a predominantly circumscribed, high-density mass. On US, metaplastic carcinoma may presents as having solid and cystic components and may be microlobulated [31, 69].
These tumors are generally poorly differentiated and ER-, PgR-, and HER2-negative, with high Ki67 and p53 positivity [4, 39]. The majority of metaplastic carcinomas are node-negative but have a high potential for metastatic spread [4]. Up to 10% of the patients present with de novo metastatic disease, and local or distant relapse is reported in >50% of the cases [70]. The prognosis is poorer than that of triple-negative invasive ductal carcinoma [67]. In the study by Hennessy et al. [71] including exclusively the squamous-cell subtype, the median OS was 37 months. From the onset of distant metastasis, survival is generally less than 1 year [5, 72]. On the other hand, well-differentiated metaplastic carcinomas, which are extremely rare, are generally associated with a good prognosis.
High-Grade Small Cell Neuroendocrine Carcinoma
This subtype is similar to neuroendocrine tumors arising in the lung. The diagnosis requires the exclusion of a nonmammary primary. Similar to other neuroendocrine tumors, neuroendocrine markers must be expressed in at least 50% of the cells. The positivity of ER and PgR inversely correlates with the degree of differentiation. HER2 is typically negative [73]. These tumors are generally associated with an aggressive disease course.
Genomic Features of Rare BC Histologies
The comprehensive evaluation of the genomic landscape of BC is one of the main goals of the current research. Only a few studies have focused on rare BC entities so far. The observation that rare BC histologies are more homogeneous at the transcriptome level than classic histologies suggests that a similar degree of homogeneity may be also found at the genomic level, thus representing a model to investigate cancer pathways (Fig. 2). Herein we summarize the most relevant data about genomic characterization of rare BC histologies.
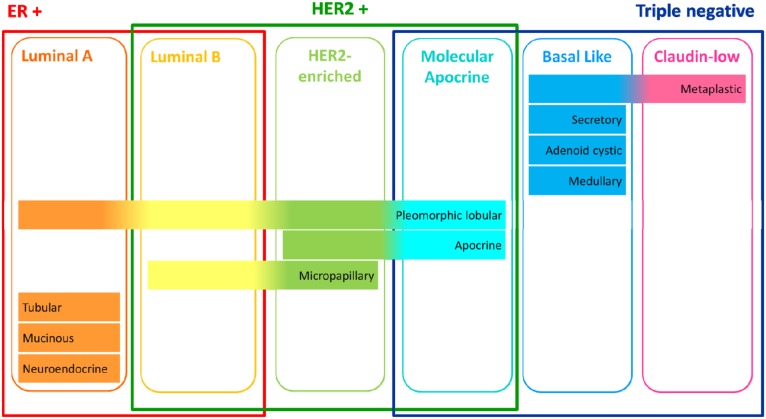
Figure 2.
Heterogeneity of breast cancer: histotypes, molecular classification, and immunohistochemical classification.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Good-Prognosis Group
A recent work evaluated the genomic profiles of 59 BC samples of 10 special types [12]. The first relevant result was that samples of the same histology presented similar degrees of gene copy number variations. In addition to that, some of the special types with the best prognosis (adenoid cystic, mucinous, and tubular carcinomas with neuroendocrine features) presented the lowest levels of gene copy number changes. Similarly, typically low-grade BC special types such as adenoid cystic, mucinous, and carcinomas presented low levels of genomic instability and simplex genomic architectural patterns.
In regard to specific aberrations selectively present or absent in rare good-prognosis histologies, some examples can be proposed. Mucinous, neuroendocrine, and adenoid cystic BC special types lack 1q gains and 16q losses, which are hallmark features of low-grade invasive ductal carcinomas, thus suggesting that pathways driving the carcinogenesis of these rare entities may be unique [12, 74, 75]. In support of this hypothesis, mucinous carcinomas of the breast lack PIK3CA and AKT1 mutations, which is in contrast with the high frequency (up to 45%) at which PIK3CA mutations occur in luminal BCs [76] (TCGA). Within the typically hormone receptor-negative good-prognosis BC special types, we can identify two main histologies for which specific fusion genes have been identified. Adenoid cystic BCs recurrently harbor the t(6;9)(q22–23;p23–24) translocation that leads to the formation of the MYB-NFIB fusion gene, which involves the MYB oncogene and the NFIB transcription factor. Indeed, a recent work reported the presence of this translocation in 12 of 13 analyzed adenoid cystic breast cancers. Interestingly, all 13 adenoid cystic BCs displayed significantly higher MYB expression levels than grade-matched and basal-like ductal carcinomas [74]. The t(12;15)ETV6-NTRK3 translocation, which is pathognomonic for secretory carcinoma, has already been described [48].
Finally, despite the fact that medullary BCs seem to share main genomic features with basal-like BCs, such as 1q and 8q gains and X losses and TP53 mutations, some genomic aberrations seem to occur more frequently in medullary BC, like cytokeratin 5/6 expression; 10p, 9p, and 16q gains; 4p losses; and 1q, 8p, 10p, and 12p amplicons [35].
Poor-Prognosis Group
The interest in deciphering specific pathways of progression of rare aggressive BC subtypes relies on the possible identification of driver aberrations that may be also involved in the progression of common histologies. From this perspective, the results of whole exome sequencing of lobular pleomorphic, micropapillary, and metaplastic BCs have been recently presented [77]. The most interesting result was represented by mutations in a gene that had not been previously described as a cancer-related gene, PYGM, in up to 30% of pleomorphic lobular cancers. This gene encodes for the muscle isoform of glycogen phosphorylase and was shown to be constantly underexpressed in BC (irrespectively of the histological or molecular subtype) compared with normal breast tissue in the TCGA database. Similar results were also obtained when looking at PYGM expression in other cancers, thus suggesting that glycogen metabolism might represent a pathway for cancer progression. TP53 and PIK3CA were extremely frequently mutated in metaplastic BCs (78% and 48%, respectively). The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is frequently aberrant in basal-like breast cancers, but PIK3CA mutations generally occur at a low rate (<10%) [78], which contrasts with what has been observed for metaplastic BCs, indicating a potential rationale to test new selective PIK3CA inhibitors in this entity. Finally, micropapillary BCs showed the typical mutation spectrum of luminal B BCs, with the most frequently mutated genes being PIK3CA, TP53, MAP2K4, and GATA3. A recently published work that explored genomic features of micropapillary BC also found that this entity does not seem to extensively differ from other luminal cancers and failed to demonstrate pathognomonic genomic features that drive micropapillary BCs [79].
Rare Histologies: Implications for Local and Systemic Therapy
Special histologies often present peculiar clinical behaviors. However, the fact that these entities can be very rare has impaired their extensive clinical evaluation. Indeed, the majority of information on outcome and treatments derives from small series and case reports. As a consequence, clear recommendations about clinical management of BC special histologies are still lacking.
The recommendations for locoregional treatments follow those of BCs of no special type. Indeed, in a study including mucinous, medullary, tubular, and invasive ductal BC, a similar rate of local failure after breast conserving therapy was observed [80]. The only exception is secretory carcinoma. Because it usually arises in childhood, breast-conserving surgery represents an issue because it would be preferable to avoid radiotherapy. In the past years, the role of axillary dissection has been questioned for the good-prognosis rare histotypes. However, because the sentinel node biopsy is nowadays a standard procedure with negligible side effects, the management of axillary lymph nodes is no longer an issue.
The adjuvant systemic therapy is more critical. The 2014 NCCN Guidelines [81] include specific treatment recommendations for the favorable tubular and mucinous histotypes. In case of HR positivity and in the absence of nodal involvement, adjuvant endocrine therapy can be avoided in case of tumor size (T) < 1 cm, should be considered for T between 1 and 3 cm, and is recommended for T ≥ 3 cm. In case of nodal involvement, endocrine therapy with or without chemotherapy is indicated. In the case of negative expression of HR, ER, and PgR status should be reassessed. If HR− is confirmed, patients should be treated as having the usual BC histology. The metaplastic cancer shares the same indications of ductal histology. The 2013 St. Gallen consensus conference simply recommends endocrine therapy for endocrine-responsive “special histological types” (cribriform, tubular, and mucinous) and cytotoxic therapy for endocrine nonresponsive special types (apocrine, medullary, adenoid cystic, and metaplastic) [9]. It is reported that adenoid cystic carcinoma may not require adjuvant chemotherapy, in case of node negativity. As previously discussed, the rarity of these tumors impedes the buildup of a robust scientific evidence. Thus, it is assumed by current international guidelines that the same chemotherapy regimens used for common BC histotypes should also be proposed in case of uncommon histologies, when indicated. Such an indication, however, reflects the lack of prognostic data and does not necessarily recapitulate the intrinsic biological chemosensitivity of different BC histotypes. As reported in this review, most of the good-prognosis endocrine-sensitive rare histotypes usually belong to the Luminal A subtype, which tends to be chemoresistant. Indeed, in a retrospective study of more than 500 BC patients treated with conventional neoadjuvant chemotherapy, the clinicopathological response of BC of special type was significantly poorer. However, despite a low rate of response, the prognosis of mucinous and apocrine BC was good [82]. Therefore, the indication to administer potential toxic chemotherapy for rare BCs with very good prognosis should be carefully evaluated by balancing potential benefits and disadvantages. From this perspective, anthracycline-free schedules could represent an option. In regard to more aggressive rare BC histotypes, no data on specific chemotherapy regimens are available; thus, the same agents used for common BCs should be proposed. However, metaplastic BC deserves specific considerations. Small retrospective studies in the neoadjuvant and metastatic setting have shown that this heterogeneous BC entity is chemoresistant to conventional chemotherapy. In a small retrospective study by Chen et al. [83], only 18% of metaplastic BC patients showed a partial response to neoadjuvant chemotherapy, and only 8% of the patients responded to first-line chemotherapy in the metastatic setting. In this series, no patient responded to anthracycline-, vinorelbine-, or cyclophosphamide-based regimens. Similarly, the M.D. Anderson Cancer Center experience reported only a 10% complete response rate in patients with metaplastic BC undergoing neoadjuvant chemotherapy [84]. Some scattered evidence suggests that metaplastic breast cancer might benefit from chemotherapy tailored on the specific metaplastic differentiation of cancer cells. This assumption derives from the observation that the clinical behavior of metaplastic breast cancer seems to reflect the behavior of sarcomas (in case of spindle cell metaplastic breast cancer) or squamous cell carcinomas (in case of epidermoid metaplastic breast cancer). Indeed, sarcomatoid metaplastic BC patients often develop pulmonary metastasis, and squamous cell metaplastic BC is commonly associated with local relapses even after local radiotherapy. Sporadic responses to ifosfamide- and anthracycline-based chemotherapy and to platinum-based chemotherapy have been reported for sarcomatoid and epidermoid metaplastic BC, respectively [40, 71, 85]. Considering the recent results from prospective trials supporting the role of platinum salts for patients with triple-negative breast cancers, there is the rationale to consider the incorporation of such drugs in the treatment of early or advanced epidermoid metaplastic breast cancer.
It is assumed by current international guidelines that the same chemotherapy regimens used for common BC histotypes should also be proposed in case of uncommon histologies, when indicated. Such an indication, however, reflects the lack of prognostic data and does not necessarily recapitulate the intrinsic biological chemosensitivity of different BC histotypes.
From a biological perspective, the observation that metaplastic cancers may be enriched in cells with stem-like features may account for their resistance to therapy and metastatic potential [86]. On the basis of recent translational studies, there may be the rationale to test selective PI3K inhibitors for this particular BC subset. Moreover, some preclinical evidence also suggests that epidermal growth factor receptor may potentially represent a target for squamous cell metaplastic breast cancers [87].
Conclusion
The management of rare breast cancer histotypes represents a real challenge in daily clinical practice. Indeed, these entities are rare, and conducting prospective studies focused on rare breast cancers is unrealistic. Moreover, rare breast cancers represent distinct entities with different clinical behavior and response to treatment, suggesting that they might also be driven by few genomic specific events. From a biological perspective, rare BC histologies may represent discrete homogeneous molecular entities, suggesting that they might be also driven by few genomic specific events. Therefore, in-depth genomic evaluation of aggressive rare histologies might allow the identification of specific cancer pathways that may also be involved in the progression of more common histologies. A more comprehensive study of the clinical and genomic aspects of rare histological subtypes is needed to provide a more complete landscape of BC disease to support a more personalized treatment decision.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Provision of study material or patients: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Collection and/or assembly of data: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Data analysis and interpretation: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Manuscript writing: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Final approval of manuscript: Maria Vittoria Dieci, Enrico Orvieto, Massimo Dominici, PierFranco Conte, Valentina Guarneri
Disclosures
The authors indicated no financial relationships.
References
- 1.Tavassoli FA, Devilee P. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs. Lyon, France: IARC Press; 2003. [Google Scholar]
- 2.Lakhani SR, Ellis IO, Schnitt SJ, et al. WHO Classification of Tumors of the Breast. Lyon, France: IARC; 2012. [Google Scholar]
- 3.Northridge ME, Rhoads GG, Wartenberg D, et al. The importance of histologic type on breast cancer survival. J Clin Epidemiol. 1997;50:283–290. doi: 10.1016/s0895-4356(96)00366-6. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA, Lee AH, Evans AJ, et al. Tubular carcinoma of the breast: Further evidence to support its excellent prognosis. J Clin Oncol. 2010;28:99–104. doi: 10.1200/JCO.2009.23.5051. [DOI] [PubMed] [Google Scholar]
- 5.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 6.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 7.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 12.Horlings HM, Weigelt B, Anderson EM, et al. Genomic profiling of histological special types of breast cancer. Breast Cancer Res Treat. 2013;142:257–269. doi: 10.1007/s10549-013-2740-6. [DOI] [PubMed] [Google Scholar]
- 13.Capella C, Eusebi V, Mann B, et al. Endocrine differentiation in mucoid carcinoma of the breast. Histopathology. 1980;4:613–630. doi: 10.1111/j.1365-2559.1980.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 14.Clayton F. Pure mucinous carcinomas of breast: Morphologic features and prognostic correlates. Hum Pathol. 1986;17:34–38. doi: 10.1016/s0046-8177(86)80152-6. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg SG, Kay S, Chitale AR, et al. Colloid carcinoma of the breast. Am J Clin Pathol. 1971;55:355–363. doi: 10.1093/ajcp/55.3.355. [DOI] [PubMed] [Google Scholar]
- 16.Tan PH, Tse GMK, Bay BH. Mucinous breast lesions: Diagnostic challenges. J Clin Pathol. 2008;61:11–19. doi: 10.1136/jcp.2006.046227. [DOI] [PubMed] [Google Scholar]
- 17.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111:541–547. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 18.Norris HJ, Taylor HB. Prognosis of mucinous (gelatinous) carcinoma of the breast. Cancer. 1965;18:879–885. doi: 10.1002/1097-0142(196507)18:7<879::aid-cncr2820180716>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Memis A, Ozdemir N, Parildar M, et al. Mucinous (colloid) breast cancer: Mammographic and US features with histologic correlation. Eur J Radiol. 2000;35:39–43. doi: 10.1016/s0720-048x(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 20.Diab SG, Clark GM, Osborne CK, et al. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17:1442–1448. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 21.Günhan-Bilgen I, Oktay A. Tubular carcinoma of the breast: Mammographic, sonographic, clinical and pathologic findings. Eur J Radiol. 2007;61:158–162. doi: 10.1016/j.ejrad.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Oakley GJ, 3rd, Tubbs RR, Crowe J, et al. HER-2 amplification in tubular carcinoma of the breast. Am J Clin Pathol. 2006;126:55–58. doi: 10.1309/E0YE-KHBP-3YYQ-YUBD. [DOI] [PubMed] [Google Scholar]
- 23.Louwman MW, Vriezen M, van Beek MW, et al. Uncommon breast tumors in perspective: Incidence, treatment and survival in the Netherlands. Int J Cancer. 2007;121:127–135. doi: 10.1002/ijc.22625. [DOI] [PubMed] [Google Scholar]
- 24.Stutz JA, Evans AJ, Pinder S, et al. The radiological appearances of invasive cribriform carcinoma of the breast. Clin Radiol. 1994;49:693–695. doi: 10.1016/s0009-9260(05)82662-5. [DOI] [PubMed] [Google Scholar]
- 25.Page DL, Dixon JM, Anderson TJ, et al. Invasive cribriform carcinoma of the breast. Histopathology. 1983;7:525–536. doi: 10.1111/j.1365-2559.1983.tb02265.x. [DOI] [PubMed] [Google Scholar]
- 26.Venable JG, Schwartz AM, Silverberg SG. Infiltrating cribriform carcinoma of the breast: A distinctive clinicopathologic entity. Hum Pathol. 1990;21:333–338. doi: 10.1016/0046-8177(90)90235-w. [DOI] [PubMed] [Google Scholar]
- 27.Righi L, Sapino A, Marchiò C, et al. Neuroendocrine differentiation in breast cancer: Established facts and unresolved problems. Semin Diagn Pathol. 2010;27:69–76. doi: 10.1053/j.semdp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 28.López-Bonet E, Alonso-Ruano M, Barraza G, et al. Solid neuroendocrine breast carcinomas: Incidence, clinico-pathological features and immunohistochemical profiling. Oncol Rep. 2008;20:1369–1374. [PubMed] [Google Scholar]
- 29.Sapino A, Papotti M, Righi L, et al. Clinical significance of neuroendocrine carcinoma of the breast. Ann Oncol. 2001;12(suppl 2):S115–S117. doi: 10.1093/annonc/12.suppl_2.s115. [DOI] [PubMed] [Google Scholar]
- 30.Ridolfi RL, Rosen PP, Port A, et al. Medullary carcinoma of the breast: A clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Liberman L, LaTrenta LR, Samli B, et al. Overdiagnosis of medullary carcinoma: A mammographic-pathologic correlative study. Radiology. 1996;201:443–446. doi: 10.1148/radiology.201.2.8888238. [DOI] [PubMed] [Google Scholar]
- 32.Karan B, Pourbagher A, Bolat FA. Unusual malignant breast lesions: Imaging-pathological correlations. Diagn Interv Radiol. 2012;18:270–276. doi: 10.4261/1305-3825.DIR.4454-11.2. [DOI] [PubMed] [Google Scholar]
- 33.Majid AS, de Paredes ES, Doherty RD, et al. Missed breast carcinoma: Pitfalls and pearls. Radiographics. 2003;23:881–895. doi: 10.1148/rg.234025083. [DOI] [PubMed] [Google Scholar]
- 34.Vu-Nishino H, Tavassoli FA, Ahrens WA, et al. Clinicopathologic features and long-term outcome of patients with medullary breast carcinoma managed with breast-conserving therapy (BCT) Int J Radiat Oncol Biol Phys. 2005;62:1040–1047. doi: 10.1016/j.ijrobp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Vincent-Salomon A, Gruel N, Lucchesi C, et al. Identification of typical medullary breast carcinoma as a genomic sub-group of basal-like carcinomas, a heterogeneous new molecular entity. Breast Cancer Res. 2007;9:R24. doi: 10.1186/bcr1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Work ME, Andrulis IL, John EM, et al. Risk factors for uncommon histologic subtypes of breast cancer using centralized pathology review in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2012;134:1209–1220. doi: 10.1007/s10549-012-2056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vranic S, Bender R, Palazzo J, et al. A review of adenoid cystic carcinoma of the breast with emphasis on its molecular and genetic characteristics. Hum Pathol. 2013;44:301–309. doi: 10.1016/j.humpath.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Glazebrook KN, Reynolds C, Smith RL, et al. Adenoid cystic carcinoma of the breast. AJR Am J Roentgenol. 2010;194:1391–1396. doi: 10.2214/AJR.09.3545. [DOI] [PubMed] [Google Scholar]
- 39.Arpino G, Clark GM, Mohsin S, et al. Adenoid cystic carcinoma of the breast: Molecular markers, treatment, and clinical outcome. Cancer. 2002;94:2119–2127. doi: 10.1002/cncr.10455. [DOI] [PubMed] [Google Scholar]
- 40.Cadoo KA, McArdle O, O’Shea AM, et al. Management of unusual histological types of breast cancer. The Oncologist. 2012;17:1135–1145. doi: 10.1634/theoncologist.2012-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson K, Grabowski J, Saltzstein SL, et al. Adenoid cystic breast carcinoma: Is axillary staging necessary in all cases? Results from the California Cancer Registry. Breast J. 2011;17:485–489. doi: 10.1111/j.1524-4741.2011.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghabach B, Anderson WF, Curtis RE, et al. Adenoid cystic carcinoma of the breast in the United States (1977 to 2006): A population-based cohort study. Breast Cancer Res. 2010;12:R54. doi: 10.1186/bcr2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peintinger F, Leibl S, Reitsamer R, et al. Primary acinic cell carcinoma of the breast: A case report with long-term follow-up and review of the literature. Histopathology. 2004;45:645–648. doi: 10.1111/j.1365-2559.2004.01957.x. [DOI] [PubMed] [Google Scholar]
- 44.Tixier H, Picard A, Guiu S, et al. Long-term recurrence of secretory breast carcinoma with metastatic sentinel lymph nodes. Arch Gynecol Obstet. 2011;283(suppl 1):77–78. doi: 10.1007/s00404-010-1669-9. [DOI] [PubMed] [Google Scholar]
- 45.Ozguroglu M, Tascilar K, Ilvan S, et al. Secretory carcinoma of the breast: Case report and review of the literature. Oncology. 2005;68:263–268. doi: 10.1159/000086782. [DOI] [PubMed] [Google Scholar]
- 46.Vieni S, Cabibi D, Cipolla C, et al. Secretory breast carcinoma with metastatic sentinel lymph node. World J Surg Oncol. 2006;4:88. doi: 10.1186/1477-7819-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mun SH, Ko EY, Han BK, et al. Secretory carcinoma of the breast: Sonographic features. J Ultrasound Med. 2008;27:947–954. doi: 10.7863/jum.2008.27.6.947. [DOI] [PubMed] [Google Scholar]
- 48.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/s1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 49.Herz H, Cooke B, Goldstein D. Metastatic secretory breast cancer. Non-responsiveness to chemotherapy: Case report and review of the literature. Ann Oncol. 2000;11:1343–1347. doi: 10.1023/a:1008387800525. [DOI] [PubMed] [Google Scholar]
- 50.Zagorianakou P, Zagorianakou N, Stefanou D, et al. The enigmatic nature of apocrine breast lesions. Virchows Arch. 2006;448:525–531. doi: 10.1007/s00428-005-0095-z. [DOI] [PubMed] [Google Scholar]
- 51.Rosen PP. Rosen’s Breast Pathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 52.Japaze H, Emina J, Diaz C, et al. “Pure” invasive apocrine carcinoma of the breast: A new clinicopathological entity? Breast. 2005;14:3–10. doi: 10.1016/j.breast.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Vranic S, Tawfik O, Palazzo J, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23:644–653. doi: 10.1038/modpathol.2010.50. [DOI] [PubMed] [Google Scholar]
- 54.Dellapasqua S, Maisonneuve P, Viale G, et al. Immunohistochemically defined subtypes and outcome of apocrine breast cancer. Clin Breast Cancer. 2013;13:95–102. doi: 10.1016/j.clbc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Montagna E, Maisonneuve P, Rotmensz N, et al. Heterogeneity of triple-negative breast cancer: Histologic subtyping to inform the outcome. Clin Breast Cancer. 2013;13:31–39. doi: 10.1016/j.clbc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Weidner N, Semple JP. Pleomorphic variant of invasive lobular carcinoma of the breast. Hum Pathol. 1992;23:1167–1171. doi: 10.1016/0046-8177(92)90035-2. [DOI] [PubMed] [Google Scholar]
- 57.Butler D, Rosa M. Pleomorphic lobular carcinoma of the breast: A morphologically and clinically distinct variant of lobular carcinoma. Arch Pathol Lab Med. 2013;137:1688–1692. doi: 10.5858/arpa.2012-0603-RS. [DOI] [PubMed] [Google Scholar]
- 58.Eusebi V, Magalhaes F, Azzopardi JG. Pleomorphic lobular carcinoma of the breast: An aggressive tumor showing apocrine differentiation. Hum Pathol. 1992;23:655–662. doi: 10.1016/0046-8177(92)90321-s. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs M, Fan F, Tawfik O. Clinicopathologic and biomarker analysis of invasive pleomorphic lobular carcinoma as compared with invasive classic lobular carcinoma: An experience in our institution and review of the literature. Ann Diagn Pathol. 2012;16:185–189. doi: 10.1016/j.anndiagpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Jung HN, Shin JH, Han BK, et al. Are the imaging features of the pleomorphic variant of invasive lobular carcinoma different from classic ILC of the breast? Breast. 2013;22:324–329. doi: 10.1016/j.breast.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Pettinato G, Manivel CJ, Panico L, et al. Invasive micropapillary carcinoma of the breast: Clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004;121:857–866. doi: 10.1309/XTJ7-VHB4-9UD7-8X60. [DOI] [PubMed] [Google Scholar]
- 62.Zekioglu O, Erhan Y, Ciris M, et al. Invasive micropapillary carcinoma of the breast: High incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44:18–23. doi: 10.1111/j.1365-2559.2004.01757.x. [DOI] [PubMed] [Google Scholar]
- 63.Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: Eighty cases of an underrecognized entity. Hum Pathol. 2001;32:583–589. doi: 10.1053/hupa.2001.24988. [DOI] [PubMed] [Google Scholar]
- 64.Günhan-Bilgen I, Zekioglu O, Ustün EE, et al. Invasive micropapillary carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;179:927–931. doi: 10.2214/ajr.179.4.1790927. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Fan Y, Lang RG, et al. Breast carcinoma with micropapillary features: Clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16:155–163. doi: 10.1177/1066896907307047. [DOI] [PubMed] [Google Scholar]
- 66.Pezzi CM, Patel-Parekh L, Cole K, et al. Characteristics and treatment of metaplastic breast cancer: Analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166–173. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 67.Jung SY, Kim HY, Nam BH, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627–637. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 68.Al Sayed AD, El Weshi AN, Tulbah AM, et al. Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncol. 2006;45:188–195. doi: 10.1080/02841860500513235. [DOI] [PubMed] [Google Scholar]
- 69.Günhan-Bilgen I, Memiş A, Ustün EE, et al. Metaplastic carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002;178:1421–1425. doi: 10.2214/ajr.178.6.1781421. [DOI] [PubMed] [Google Scholar]
- 70.Yerushalmi R, Hayes MM, Gelmon KA. Breast carcinoma: Rare types: Review of the literature. Ann Oncol. 2009;20:1763–1770. doi: 10.1093/annonc/mdp245. [DOI] [PubMed] [Google Scholar]
- 71.Hennessy BT, Krishnamurthy S, Giordano S, et al. Squamous cell carcinoma of the breast. J Clin Oncol. 2005;23:7827–7835. doi: 10.1200/JCO.2004.00.9589. [DOI] [PubMed] [Google Scholar]
- 72.Tse GM, Tan PH, Putti TC, et al. Metaplastic carcinoma of the breast: A clinicopathological review. J Clin Pathol. 2006;59:1079–1083. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alkaied H, Harris K, Azab B, et al. Primary neuroendocrine breast cancer, how much do we know so far? Med Oncol. 2012;29:2613–2618. doi: 10.1007/s12032-012-0222-z. [DOI] [PubMed] [Google Scholar]
- 74.Lacroix-Triki M, Suarez PH, MacKay A, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282–298. doi: 10.1002/path.2763. [DOI] [PubMed] [Google Scholar]
- 75.Wetterskog D, Lopez-Garcia MA, Lambros MB, et al. Adenoid cystic carcinomas constitute a genomically distinct subgroup of triple-negative and basal-like breast cancers. J Pathol. 2012;226:84–96. doi: 10.1002/path.2974. [DOI] [PubMed] [Google Scholar]
- 76.Kehr EL, Jorns JM, Ang D, et al. Mucinous breast carcinomas lack PIK3CA and AKT1 mutations. Hum Pathol. 2012;43:2207–2212. doi: 10.1016/j.humpath.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Dieci MV, Lefebvre C, Scott V et al. Whole exome sequencing of rare aggressive breast cancer histologies reveal novel pathways for breast cancer progression. Paper presented at: 2013 San Antonio Breast Cancer Symposium, December 10–14, 2013; San Antonio, TX. Abstract 798. [Google Scholar]
- 78.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Natrajan R, Wilkerson PM, Marchiò C, et al. Characterization of the genomic features and expressed fusion genes in micropapillary carcinomas of the breast. J Pathol. 2014;232:553–565. doi: 10.1002/path.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vo T, Xing Y, Meric-Bernstam F, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194:527–531. doi: 10.1016/j.amjsurg.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 81.NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines, Breast Cancer v1.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Accessed February 27, 2014.
- 82.Nagao T, Kinoshita T, Hojo T, et al. The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: The relationship between the outcome and the clinicopathological characteristics. Breast. 2012;21:289–295. doi: 10.1016/j.breast.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Chen IC, Lin CH, Huang CS, et al. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130:345–351. doi: 10.1007/s10549-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 84.Hennessy BT, Giordano S, Broglio K, et al. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- 85.Brown-Glaberman U, Graham A, Stopeck A. A case of metaplastic carcinoma of the breast responsive to chemotherapy with ifosfamide and etoposide: Improved antitumor response by targeting sarcomatous features. Breast J. 2010;16:663–665. doi: 10.1111/j.1524-4741.2010.00975.x. [DOI] [PubMed] [Google Scholar]
- 86.Hennessy BT, Gonzalez-Angulo A-M, Stemke-Hale K, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura F, Iwaya K, Kawaguchi T, et al. Epidermal growth factor-dependent enhancement of invasiveness of squamous cell carcinoma of the breast. Cancer Sci. 2010;101:1133–1140. doi: 10.1111/j.1349-7006.2010.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]