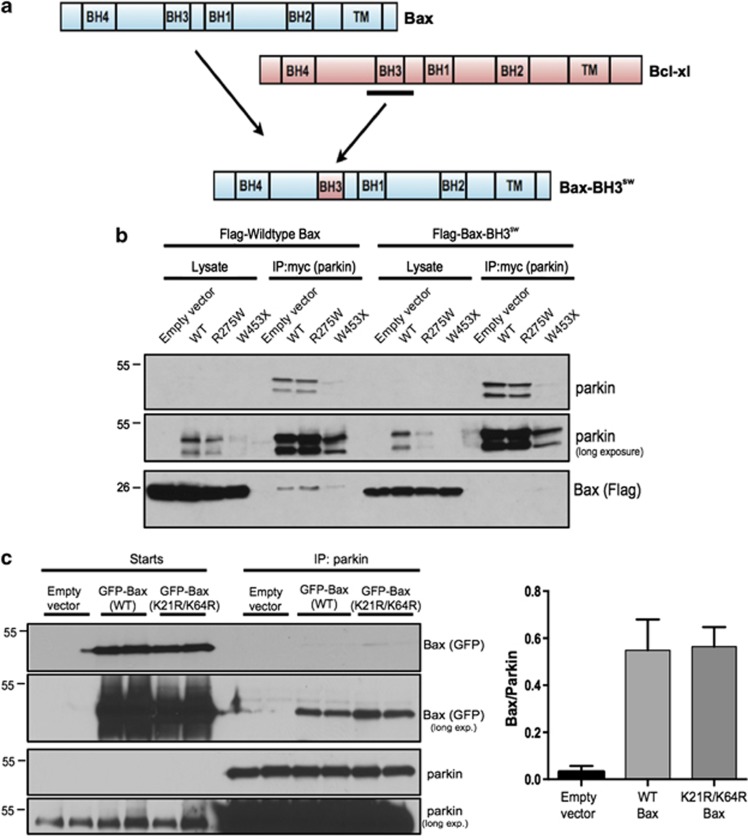
Figure 5.
Swapped Bax BH3 domain causes reduction in the parkin-Bax interaction. (a) Schematic of Bax and Bcl-xl structures and the engineered construct with the BH3 domain swapped with the BH3 domain of Bcl-xl (Bax-BH3sw). (b) Flag-tagged wild-type Bax or Bax-BH3sw were transiently co-transfected in HEK cells along with empty vector, myc-tagged wild-type parkin (WT) or mutant parkin (R275W and W453X). Whole-cell lysates were subjected to IP of parkin using a myc-tag antibody and whole-cell lysates and IPs were probed for Bax using a Flag-tag antibody to visualize co-immunoprecipitation along with parkin to demonstrate pulldown. (c) GFP-tagged WT or K21R/K64R Bax was transiently transfected in CHO cells stably expressing parkin (CHO-Parkin). Whole-cell lysates were subjected to IP using a parkin antibody, and starting lysates and IPs were probed for Bax using a GFP antibody to visualize co-immunoprecipitation along with parkin to demonstrate pulldown. For quantification, band intensities of WT and mutant Bax were normalized to levels of parkin pulldown. All bars represent mean±S.D., n=5