Abstract
Optical imaging of luciferage gene expression has become a powerful tool to track cells and viruses in vivo in small animal models. Luciferase imaging has been used to study the location of infection by replication-defective and replication-competent viruses and to track changes in the distribution of viruses in mouse models. This approach has also been used in oncolytic studies as a non-invasive means to monitor the growth and killing of tumor cells modified with luciferase genes. In this chapter, we describe the techniques used for luciferase imaging as have been applied to track replication-defective and replication-competent adenoviruses in mouse and hamster models of oncolysis and virus pharmacology. Although these methods are simple, the process of obtaining accurate luciferase imaging data has many caveats that will be discussed.
Keywords: Luciferase, in vivo imaging, luminescence, luciferin, adenovirus
1. Introduction
The technologies available to the basic scientist to track and localize viruses and tumor cells have historically been quite primitive. In most cases, virus and cell trafficking has been assessed by the use of terminal assays in which the animal must be sacrificed and the cells or viruses are tracked after the animal is “taken apart” either at the organ level or in tissue sections. These “grind and find” assays are quite laborious requiring that one actually section the whole animal to be certain of the tissue localization of the virus to ensure that all sites are observed and unexpected localization sites are not missed. Furthermore, these terminal assays obviate the ability to perform kinetic studies in one animal over many time points.
Given these difficulties, non-invasive and non-terminal virus and cancer cell tracking was needed. One approach that partially satisfies this need is to "arm" viruses or cancer cells with reporter genes that can be detected by imaging. Reporter genes encode proteins that are easily detected in cells and in intact animals with sensitive imaging systems. The most used reporter genes include β-galactosidase, luciferases, green fluorescent protein (GFP) and its varied color derivatives, and alkaline phosphatase. Of these, luciferase and fluorescent proteins can be used to varying degrees for optical imaging at visible wavelengths of light in small animals. One can also use reporter genes such as thymidine kinase or the sodium iodide symporter for higher energy PET and SPECT imaging in small animals, large animals, and in humans.
For most researchers, optical imaging is simpler and more easily obtainable in the laboratory setting than radioactive imaging for PET or SPECT. Since PET and SPECT imaging are the subject of another chapter, they will not be discussed further here. One can in some cases directly image reporters like GFP and other fluorescent proteins in living animals. In practice, high background fluorescence and scatter in the green, red, and far red wavelengths makes the "noise" of imaging too high to easily detect most current fluorescent proteins in vivo (Blum et al., 2004; Adams et al., 2007). Newer far red fluorescent proteins to date are still difficult to image in mice (unpublished observations), but future near-infrared fluorophores may circumvent this difficulty.
Given these issues, luciferase imaging is arguably the best choice for non-invasive, inexpensive, and non-radioactive imaging in small animals. Given this we have "armed" replication-defective and replication-competent adenovirus serotype 5 (Ad5) viruses with luciferase and GFP-luciferase reporter genes to track 1) sites of infection, 2) persistence of infection, 3) spread of virus, and 4) elimination of virus due to immune responses (Blum et al., 2004; Mok et al., 2005; Hofherr et al., 2007; Hofherr et al., 2008; Shashkova et al., 2008; Weaver and Barry, 2008; Doronin et al., 2009; Shashkova et al., 2009; Weaver et al., 2009; Chen et al.). In addition, one can monitor immune responses against these proteins in immunocompetent mice ((i.e. H-2d-restricted T cells in BALB/c mice vs. GFP, antibodies against luciferase (Weaver and Barry, 2008; Weaver et al., 2009)). These virus persistence-immune response studies of course cannot be performed in immunodeficient models using human tumor xenografts.
With these applications in mind, below we provide the simple protocol for imaging codon-optimized firefly luciferase with its substrate luciferin after Ad5 infection. Similar approaches can be applied for other luciferases (e.g. Gaussia luciferase, Renilla luciferase). These other luciferases use coelenterazine rather than luciferin as a substrate so injections of substrate and timing of imaging are different. In our hands, the coelentarazine substrate is less soluble and does not distribute as well as luciferin, so can be more difficult to use.
2. Materials
2.1. Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco/BRL, Bethesda, MD)
2.2. Luciferin
(Molecular Imaging Products Company, Bend, OR) diluted to 20 mg/ml in DPBS, filter sterilize using a 0.22 micron filter. Aliquot and store at −80C. Protect from light exposure and avoid repeated freeze-thaws.
3. Methods
3.1. Animal Preparation for Luciferase Imaging
Appropriate animal protocol and biosafety approvals must be obtained before performing these experiments. Investigators should apply appropriate biosafety containment in instruments and rooms whenever imaging is performed. The following is an example of luciferase imaging after intramuscular (i.m.) injection of a replication-defective Ad5 vector (Ad-Luc).
Dilute Ad-Luc in DPBS to 2e11 virus particles (vp)/ml.
Using a 0.3 ml 29 G ½” syringe inject 0.025 ml of virus into each quadriceps of the mouse.
After 24 hrs. place the mouse in an isofluorane induction chamber (3–5%).
Once the animal is under sedation, inject 0.2 ml of luciferin (20 mg/ml in sterile DPBS) intraperitonealy (i.p.). Inject the luciferin substrate 5 – 10 minutes prior to image capture.
Working quickly, transfer the mouse to the imaging system and maintain sedation with 1–3% isofluorane. We have found that mice can be maintained for extended lengths of time without risk to the animals at these isofluorane levels.
Image the mouse dorsally using a 10 minute exposure with 4×4 binning or 1×1 binning depending on signal intensity.
Note: Alternative anesthesia. If isofluorane is not available the mice can be anesthesized with ketamine and xylazine. Dilute the ketamine (27.77 mg/ml) and xylazine (1.11 mg/ml) in sterile dH20 and inject 0.1 ml i.p. into a 20 – 25 g mouse using a 1.0 ml 26G 5/8” syringe. The mouse will be sedated for approximately 30 minutes.
3.2. Image Capture Using Lumazone Imager
Note: Each imaging system will require different steps in order to obtain both the chemiluminescent luciferase image as well as the brightfield white image of the animal itself. Below is provided the steps for a Lumazone (Roper) imager.
Make sure cap is on the white light source in the imaging cabinet
Open Lumazone software. The ‘Lumazone Analyzer’ box should display:
- Select ‘Configure’
- Choose ‘Luciferin’ from Experiment Type drop down (This only affects how the image is titled after multi-channel acquire is chosen and imaging is complete)
- Select ‘Chemiluminescence’ box
- Select ‘Brightfield’ box
- Select ‘OK’
- Select Focus/Exposure
- Select on ‘Chemi’ button (for chemiluminescence)
- Make sure ‘Adjust Exp for Binning’ box is checked
- Choose binning setting
- Set ‘Exp Pvw’ time (should show in MM:ss:mmm)
- If ‘Adjust Exp for Binning’ box is not checked, you will also need to set the ‘Exp Acq’ time
- Select ‘BF’ (for brightfield)
- Select ‘Live’ to check and/or modify the focus of the image
- Select ‘Close’
- Select ‘Multichannel Acquire’ in the ‘Lumazone Analyzer’ box to begin taking the image
- Once the image has been captured, Select on the ‘Best Fit Display Range or Contrast Equalization’ button to bring the picture into focus – complete for each image.
- 2 images will display. One image is the BF / whitelight image. One image is the chemiluminescence image.
- Save the images
3.3. Image Display
The bright light and chemiluminescent images are captured as gray scale images (Figure 1). In order to create an image that is more aesthetically pleasing an easier to interpret by eye, the images can be pseudo-colored and overlaid onto a white light image of the animals.
Load the experiment. This will open up an image of the light emitted in vivo and a white light image of the animals (Figure 1A).
Select the Calibrated LUT function on the analyze toolbar and select the image you want to calibrate. When prompted select “No” in order to create a new image for calibration. This will bring up an entirely new image window and will not alter the original image (Figure 1B).
Two windows will open when the calibrated image is created. Select Best Fit in the Display Range Window and use the LUT range to adjust the image and remove low level background signal (Figure 1C)
Select OK on the Calibrated LUT window and Yes to placing a calibration bar on the image.
Modify the calibration bar as needed and place in an area of the image that does not show signal (Figure 1D).
Make changes to the font and overall appearance of the calibration bar and select OK. This will create a pseudo-colored image with a calibration bar.
In order to show the position of the signal relative to the animal, an overlayed image can be created.
Select Overlay Images and Yes the image is calibrated. Select the calibrated image and then the white light image of the animals and an overlayed image will be created (Figure 1E)
Figure 1. Bright light and chemiluminescent images.
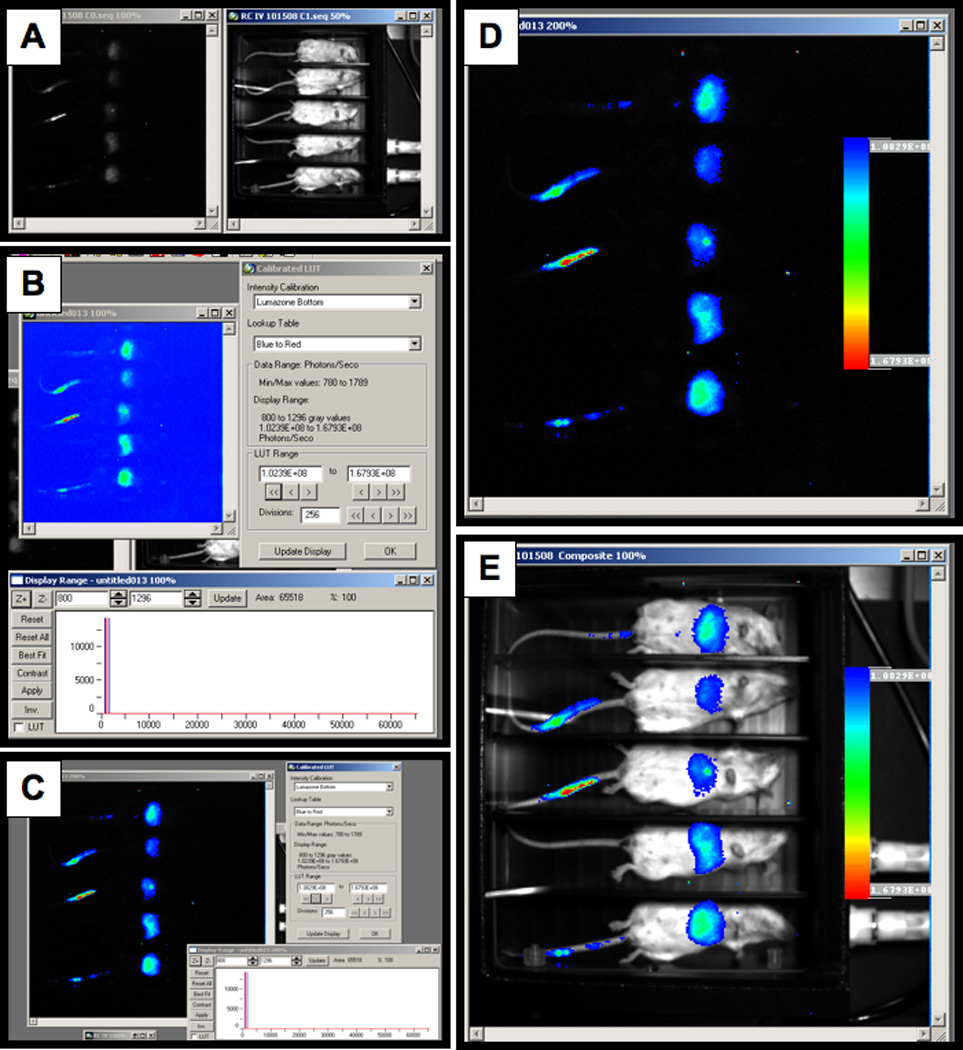
A) Image of the light emitted in vivo and a white light image of the animals. B) Calibrated LUT function. C) Best Fit in the display range window to remove low level background signal. D) Background area selection. E) Image overlay selection.
3.4. Image Data Analysis
After capturing an image data analysis need to be performed. There are several ways to capture and analyze the data. The primary issue of concern is light contamination, either from outside the imaging chambers, from other animals or from background signal inherent to all electronics. The following is an example of image analysis performed using the Lumazone Imaging System.
Capture an image as previously described.
Load the experiment to be analyzed.
The first thing that needs to be done is to subtract the background signal. This can be done using gray values without intensity calibration or using photons as determined by intensity calibration.
Define an area of interest (AOI) in the image outside of the area where the mice are imaged Edit>New AOI (Figure 2A).
In order to define background levels the AOI background signal levels need to be determined. Open up count/size: Measure>Count/Size. Select the range of intensity to measure. For this procedure the range should be the full scope of the range. For the Lumazone this range is 0 to 65535. Select the measurements to be determined using the Count/Size function: Measure>Count/Size>Measure>Select Measurements. In this case, select the Den./Inten. (mean). Select count on the Count/Size function. To view the value either click on the AOI or select Measure>Count/Size>Measure>View>Object Attributes. In this example the Den./Inten. (mean) = 811.51459.
Once the background signal levels have been determined they can be subtracted from the image. Open up image operations: Process>Operations. Check the following: Operation = Subtract, 2nd operand = Number, Put result in = New Image. Enter the background signal (811.51459), deselect the AOI, and select apply (Figure 2B). Select the Best Fit icon to view the best display range and contrast equalization.
Create an AOI over the area of the image to be quantitated. In the Count/Size function select the measurements to acquire. In general, the Den./Inten. (sum) values are the most representative of the signal being measured. However, in some cases the Den./Inten. (mean) may be more informative.
Depending on the values desired gray values or converted photons/second can be reported. Select (none) in the intensity calibration if gray values are desired or select the calibration curve that was established when the unit was installed. In this case “Lumazone Bottom” will give values reported as photons/second by converting gray values to photons using the calibration curve. Select Apply.
In the Count/Size function select Count and Measure. Double click on the AOI to view the object attributes. In this example there are 9.01972e9 photons/sec emitted from the AOI (Figure 2B).
Figure 2. Quantitating luciferase photon output.
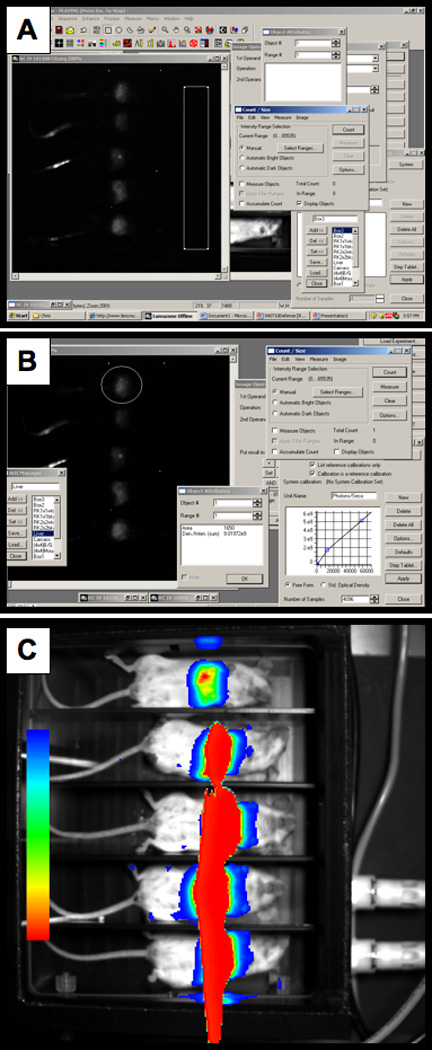
A) Defining an area of interest. B) Applying background correction.
4. Notes
Although the basic procedure of image capture during luciferase imaging is relatively simple, luciferase imaging is a dynamic procedure. As with all catalytic reactions the signal is constantly changing. Luciferin, for example, is being degraded by the enzyme lucferase. Therefore, the procedures should be performed in a consistent and timely manner. Also, another issue of concern is how long of an exposure is needed. This will change from experiment to experiment and will be dependent upon many factors including how much virus was administered, the route, and even the position of the animal. Over-exposure as shown in Figure 2C can lead to a loss of valuable data. In this example the exposure time should be reduced. Another issue is that light can be reflected off other objects, but still represents signal from the animal (Figure 2C). A chamber that has dividers to separate the animals during luciferase imaging will help eliminate this problem.
Acknowledgements
We would like to thank all past and present Barry laboratory members for their experiences in imaging that have been distilled here. This work has been supported by the Muscular Dystrophy Association, the Ralph C. Wilson Foundation, the Propionic Acidemia Foundation, R01 AI06709, R01 AI065304, R01 CA136945-01A2, and by the NIH P50 CA91956 Prostate Cancer SPORE grant at the Mayo Clinic.
References
- 1.Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, Hirschi K, Mawad ME, Barry MA, Sevick-Muraca E. Comparison of visible and near-infrared wavelength excitable fluorescent dyes for molecular imaging of cancer. J Biomed Optics. 2007;12 doi: 10.1117/1.2717137. 024017-024011-024019. [DOI] [PubMed] [Google Scholar]
- 2.Blum JS, Temenoff JS, Park H, Jansen JA, Mikos AG, Barry MA. Development and characterization of enhanced green fluorescent protein and luciferase expressing cell line for non-destructive evaluation of tissue engineering constructs. Biomaterials. 2004;25:5809–5819. doi: 10.1016/j.biomaterials.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Chen CY, May S, Barry MA. Targeting Adenoviruses with Factor X-Single Chain Antibody Fusion Proteins. Hum Gene Ther 2010. 2010 Mar 23; doi: 10.1089/hum.2009.190. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofherr SE, Mok S, Gushiken FC, Lopez JA, Barry MA. Polyethylene Glycol Modification of Adenovirus Reduces Platelet Activation, Endothelial Cell Activation, and Thrombocytopenia. Human Gene Therapy. 2007;18:837–848. doi: 10.1089/hum.2007.0051. [DOI] [PubMed] [Google Scholar]
- 6.Hofherr SE, Shashkova EV, Weaver EA, Khare R, Barry MA. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther. 2008;16:1276–1282. doi: 10.1038/mt.2008.86. [DOI] [PubMed] [Google Scholar]
- 7.Mok H, Palmer DJ, Ng P, Barry MA. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer research. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 9.Shashkova EV, May SM, Doronin K, Barry MA. Expanded Anticancer Therapeutic Window of Hexon-modified Oncolytic Adenovirus. Mol Ther Online. 2009 Sep 15; doi: 10.1038/mt.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver EA, Barry MA. Effects of Shielding Adenoviral Vectors with Polyethylene Glycol (PEG) on Vector-specific and Vaccine-mediated Immune Responses. Hum Gene Ther. 2008;19:1369–1382. doi: 10.1089/hum.2008.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, Ng P, Sastry KJ, Barry MA. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE. 2009;4:e5059. doi: 10.1371/journal.pone.0005059. [DOI] [PMC free article] [PubMed] [Google Scholar]


