Abstract
The development of ultrasound contrast agents with excellent tolerance and safety profiles has notably improved liver evaluation with ultrasound (US) for several applications, especially for the detection of metastases. In particular, contrast enhanced ultrasonography (CEUS) allows the display of the parenchymal microvasculature, enabling the study and visualization of the enhancement patterns of liver lesions in real time and in a continuous manner in all vascular phases, which is similar to contrast-enhanced computed tomography (CT) and contrast-enhanced magnetic resonance imaging. Clinical studies have reported that the use of a contrast agent enables the visualization of more metastases with significantly improved sensitivity and specificity compared to baseline-US. Furthermore, studies have shown that CEUS yields sensitivities comparable to CT. In this review, we describe the state of the art of CEUS for detecting colorectal liver metastases, the imaging features, the literature reports of metastases in CEUS as well as its technique, its clinical role and its potential applications. Additionally, the updated international consensus panel guidelines are reported in this review with the inherent limitations of this technique and best practice experiences.
Keywords: Ultrasound, Contrast enhanced ultrasound, Ultrasound contrast agent, Colorectal cancer, Colorectal liver metastases, Magnetic resonance imaging, Computed tomography, 18F-fluorodeoxyglucose positron emission tomography, Intraoperative ultrasound
Core tip: Contrast enhanced ultrasound has completely changed the ultrasound liver imaging of the colorectal cancer patient, notably increasing its sensitivity and accuracy in metastases detection. Clinical studies have reported that the use of a contrast agent enables the visualization of more metastases with significantly improved sensitivity and specificity compared to baseline-ultrasound. Furthermore, studies have shown that contrast enhanced ultrasound yields sensitivities comparable to computed tomography. In this review, we describe the state of the art of this technique.
INTRODUCTION
In patients with diagnosed colorectal cancer (CRC), the early detection of liver metastasis, is of fundamental importance for achieving cancer control[1-3]. This is because while limited synchronous or metachronous colorectal liver metastases (CRLM) are primarily treated with surgical resection, they can also be treated with preoperative chemotherapy and irradiation or newer ablation techniques[4-8]. The treatment of CRLM increases the disease free time and improves the overall survival.
The sensitivity of ultrasound (US), for metastasis detection is reported in the literature as low and variable, ranging from 50%-76%[1-3]. It is well known that US is a technique that is dependent on both the operator and the patient, and operator expertise and the patient’s habitus (fat and body mass index) and intestinal gas variably influence the accuracy of US. However, the major limit of US is its low imaging contrast between liver lesions and the liver parenchyma. In particular, isoechoic metastases are generally difficult to detect as they have similar acoustic impedance to the surrounding parenchyma, and hyperechoic metastases are difficult to differentiate from hemangiomas. Bipat et al[9] recommends that US should only be used to distinguish patients with diffuse CRLM, who are no longer eligible for curative treatment, from those with few metastases or other liver lesions, who require further imaging. However, with the introduction of contrast enhanced US (CEUS), a new era has begun. In a similar fashion to contrast-enhanced multidetector computed tomography (CT) and magnetic resonance imaging (MRI), CEUS identifies more liver metastases, with reported accuracy rates as high as 90%[10-12] with both high sensitivity for detecting CRLM and high specificity for characterizing focal liver lesions. Conversely, US is notorious for its low specificity, and it is mostly unable to differentiate and characterize benign liver tumors, which have a prevalence as high as 20%[13,14]. The efficacy of CEUS in detecting liver metastases has been recognized by review papers and by recent international guidelines[1,15-19]. Nevertheless, caution is required, as with the implementation of all new techniques and procedures, and further detailed analysis is necessary before liver CEUS for CRLM detection can become integrated appropriately into the current diagnostic algorithms. In this review article, we aim to present the state of the art of the CEUS technique and its application in the detection of CRLM, along with a detailed analysis of the current literature. We also compare the CEUS technique with methodologies that have the highest diagnostic performance, such as CT, MRI and 18F fluorodeoxyglucose positron emission tomography (FDG-PET). Additionally, the diagnostic value of CEUS in patients with colorectal cancer is discussed in the context of guidelines issued by international associations[15] together with its limits and drawbacks.
LITERATURE RESEARCH
We performed a systematic literature search using the MEDLINE, EMBASE and COCHRANE databases, which we last accessed on July 2013. The MeSH headings used were as follows: CEUS, colorectal cancer, liver metastases, ultrasound, CT, MRI, and PET-CT. We limited the electronic search to English-language papers, and we reviewed the abstracts to find the relevant information, which were assessed in detail in the full text. Additionally, we reviewed the references of the papers we examined to identify other relevant reports.
NOTES ON THE STATE OF THE ART OF THE IMAGING APPROACH FOR CRLM DETECTION
Most authors recommend a multi-modality strategy for CRLM because no single modality can accurately detect all metastases[4,20].
Selection for surgical treatment of CRLM requires that the imaging criteria show complete resectability, which is based on the exact number, regional distribution, size and volume of the metastases and also the volume of the remaining liver[4]. CT is capable of accurately performing this task, and at present, CT is the mainstay for CRLM diagnosis because it offers high-resolution imaging (sub-millimeter) and reformatted images, which may enable the detection of small metastases. Furthermore, the delineation of the segmental localization of metastases by imaging the hepatic arterial and portal venous anatomy and the accurate volumetric measurement of tumor and normal liver achieved with CT are both crucial in the surgical planning of CRLM resection[4]. The reported sensitivity of contrast enhanced CT for liver metastases is 68%-85%[21-25]. Despite the high resolution of CT liver imaging, some reports have shown that this modality may still miss up to 20% of liver metastases[22,26]. Additionally, CT has several important drawbacks, including patient allergies to iodinated contrast agents, renal damage in patients with impaired renal clearance and the use of ionizing radiation.
The 2012 Dutch guidelines for the staging of CRC[27] reported a detailed, systematic survey on the imaging modalities used in all Dutch hospitals, including academic and tertiary institutions. In 52 hospitals (78.8% of the total surveyed), the first modality of choice for CRLM detection was CT, while US was the first modality of choice in 12 hospitals (18.2%). The second choice was US in 34 hospitals (51.5%) and CT in 11 hospitals (16.7%). MRI, FDG-PET and FDG-PET/CT were not frequently used as first or second choice modalities. The paper noted that in their detailed survey, only one hospital (3.2%) occasionally used contrast agents during ultrasound. The Dutch guidelines indicate either CT or MRI as the first choice for CRLM detection in staging[28].
MRI may have higher sensitivity rates than CT in CRLM[29-33], reported 70%-98%[24,34,35], and in rectal cancer, MRI is used for local staging[34,36]. Nonetheless, the use of MRI is limited due to a relative lack of operator expertise, its limited availability and high costs. Furthermore MRI cannot be performed in patients with claustrophobia, pacemakers, cardiac defibrillators, cochlear implants and ferromagnetic foreign bodies. Literature reports assessing different types of MRI contrast agents; non-specific gadolinium and liver-specific, hepato-biliary contrast agents Gd-BOPTA or reticulo-endothelial contrast agents SPIO, have showed higher diagnostic efficacy for SPIO[32,37-39]. Conversely, another study showed equal sensitivity between SPIO-enhanced MRI and Gd-BOPTA-enhanced MRI[40,41]. Some reports have claimed that the new diffusion weighted MRI techniques yield improved accuracy[42-44]. Recently, Frankel et al[45] reported that in their institution, the surveillance and staging imaging is performed with contrast-enhanced CT scans, and in cases where indeterminate lesions are encountered in the liver, MRI may then be used for their better characterization and to rule out the presence of any other lesions.
18F fluorodeoxyglucose positron emission tomography (FDG-PET), in combination with CT (PET-CT), has recently become more available, despite its very high cost, and some authors have reported it to be the most accurate modality available[9,46,47]. In fact CRC, FDG-PET and FDG PET/CT are accurate in detecting additional hepatic and extrahepatic metastases with a sensitivity of 90%-94.6%, often leading to upstaging and affecting management[9,38,47,48].
TECHNICAL ASPECTS OF CEUS
US contrast agents
The introduction and potential applications of US contrast agents represent a promising innovation that may radically change ultrasound examinations. US contrast agents (UCA) enable the display of the parenchymal microvasculature and the enhancement patterns of the liver lesions during all vascular phases in real time and in a continuous manner. This is unlike the pre-determined, fixed time points of contrast circulation, specifically, the arterial, portal venous, late and postvascular phases, which are used in contrast enhanced CT and MRI. The other important advantage of UCAs is that their circulation is confined within the vascular space, whereas CT and MRI contrast agents rapidly leave the blood and to pass into the extravascular space[15,49]. For this reason, UCAs persist in the liver much longer than the contrast agents used in CT and MRI. As they have a scanning time of approximately 5 min, UCAs allow the systematic scanning of the entire liver. The enhancement dynamics of lesions can be identified with a higher temporal resolution by CEUS than CT and MRI, and full operator control is possible.
US contrast agents are microbubbles filled with gas and covered by a shell[15,49]. As their size is comparable to red blood cells, UCAs remain in the blood pool for approximately 5 min and can depict both the macrovasculature and the microvasculature. Presently, the following three UCAs are most commonly used[15]: (1) SonoVue® (sulfur hexafluoride with a phospholipid shell) Bracco SpA, which was introduced in 2001 in Milan, Italy, and is licensed in Europe, China, India, South Korea, Hong Kong, New Zealand, Singapore and Brazil; (2) Definity®/Luminity® [octafluoropropane (perflutren) with a lipid shell], which was introduced in 2001 by Lantheus Medical, Billerica, MA, United States, and is licensed in Canada and Australia; and (3) Sonazoid® (perfluorobutane with a phospholipid shell: hydrogenated egg phosphatidyl serine), which was introduced in 2007 by Daiichi-Sankyo, GE Tokyo, Japan, and is licensed in Japan and South Korea. Although they have different chemical compositions, they all have a similar behavior with regard to liver enhancement. The exception is Sonazoid®, which presents a “postvascular phase” whereupon after disappearing from the vascular pool, it persists for several hours in the liver and spleen, where it is phagocytized by Kupffer cells[15].
UCAs are considered safe by the guidelines[15], and no cardio-, hepato- or nephrotoxicity have been reported. In comparison with CT and MRI contrast agents, UCAs have a very low incidence of hypersensitivity events, and no deaths were reported in a series of over 23000 patients[50]. Therefore, if the examination is unsatisfactory, the injection can be repeated shortly afterwards.
EXAMINATION TECHNIQUE
CEUS examination for CRLM detection starts with the traditional B-mode US for an initial evaluation of the liver morphology and changes, such as steatosis or cirrhosis, with the eventual detection of lesions, including cysts, hemangiomas, fatty sparing and other suspicious hypoechoic lesions.
For CEUS, the ultrasound device has a contrast-specific US mode that is able to form images with the cancellation of the US signals from tissue and using only the signals from the microbubbles to generate the image[15]. To achieve this, the acoustic pressure is kept low. Accordingly, a low mechanical index (MI) of 0.3-0.05 and a low gain are used to avoid the generation of signals from tissues harmonics and to minimize the disruption of the microbubbles due to the pressure of the acoustic waves. Some authors also advise placing the patient on the left decubitus because it is claimed that in this position, the liver moves closer to the transducer in the subcostal site by 1 or even 2 cm[51,52]. This helps to overcome the limited penetration of US in low MI imaging. However, with current modern devices, image quality is markedly improved, even with very low MI (0.05). Additionally, the adequate cancellation of signals from tissues is achieved, with the nearly complete disappearance from the image of the parenchymal liver structures (the screen is nearly black). The image appears with the arrival of the UCA, which provides strong reflectors that mark the macro and micro-vascular structures. In real time, two contemporary images are available for analysis, one corresponding to baseline-US and another to a low-mechanical index examination.
With the use of Sonovue® UCA, generally a 2.4 mL bolus is administered with a fine catheter (20-guage), and this is followed by 10 mL flush of saline. The three phases of liver contrast enhancement are named in a similar fashion to CT and MRI scanning of the liver and are as follows: the arterial phase, in which the supply from hepatic artery arrives first and is predominant at 10 to 35 s after injection; the portal phase follows and becomes predominant between 45 to 120 s; and, finally, the late phase occurs after 120 s[52]. As in CT and MRI, the portal and late phases are the most useful vascular phases for detecting CRLM. The examination is recorded as a whole or in the form of sequential clips during the arterial, portal and late phases for later analysis, which can be performed on workstations.
CEUS IMAGING FEATURES OF LIVER METASTASES
CEUS characterizes focal liver lesions in a similar fashion to contrast enhanced CT and MRI. Specifically, due to the characteristics of the appearance of UCA during the vascular phases, specific contrast-enhancement patterns are obtained for different liver lesions. By differentiating metastases from other liver lesions according to contrast enhancement patterns, such as in CT and MRI, CEUS may offer a high specificity for CRLM diagnosis. This is an absolute necessity in the context of the high prevalence of benign liver tumors, which has been reported to be more than 20% in autopsy studies[13,14]. Furthermore, studies have reported that 25%-50% of lesions smaller than 20 mm[53,54] and up to 80% of those smaller than 10 mm are benign[55].
CRLM are hypovascular and appear in CEUS images with a similar presentation to CT and MRI. During the arterial phase, metastases sometimes present as variable contrast enhancements that are usually tenuous and peripheral. This is because all liver metastases, either hypervascular or hypovascular, are supplied predominantly by arterial blood.
In the portal and delay phases, dark defects with stark contrast in the enhanced liver indicate hypovascular CRLM. During these phases, hypoenhancement is characteristic of and common to all metastases, regardless of eventual enhancement in the arterial phase because the liver tissue retains the UCA, while the metastases present a rapid and marked “washout”. The observed hypoenhancement could also be due to the absence of portal supply to metastases and hence a lower vascular volume in the metastases compared with the liver parenchyma[56]. Incidental benign focal liver lesions can also present with hypoenhancement at CEUS and thus the careful evaluation of any lesion is required when the liver is examined for the first time. Rare false positive results have been reported with hypoenhancing lesions, such as abscesses or necrosis, old fibrous FNH, granulomas and inflammatory pseudotumors[15].
Rare cystic metastases can be differentiated from non-neoplastic complex cysts with CEUS by the evidence of vascular flow in thickened cyst walls or in mural nodules[52].
LITERATURE REPORTS ON CEUS EFFICACY FOR CRLM DETECTION
Presently, CEUS is a promising tool that has dramatically increased the capability of US for characterizing liver lesions. Accordingly, it has the potential to be incorporated into the diagnostic algorithm of CRLM and could possibly replace US.
A number of studies (Table 1) have reported that CEUS has a considerably high sensitivity of up to 80%-90% in detecting liver metastases, and it is therefore comparable to CT[52]. Furthermore, some reports have shown that CEUS is especially sensitive for metastases smaller than 10 mm and can improve the sensitivity of US by up to an additional 50% (Figure 1)[51,57].
Table 1.
Clinical studies of contrast enhanced ultrasound efficacy for liver metastases detection, in comparison with ultrasound
| Ref. | patients (n) | Standard of reference |
Sensitivity |
Specificity |
||
| US | CEUS | US | CEUS | |||
| Itabashi et al[62], 2013 | 454 | Pathology | Intra-operative CEUS found 71 CRLMs that were not detected at preoperative imaging | |||
| Muhi et al[60], 2011 | 106 | Histology, follow up CT, SPIO/ GdEOB MRI | - | 73 | - | - |
| Cantisani et al[59], 2010 | 110 | IOUS, CT, follow up CT, MRI | 71.6 | 95.8 | 60.0 | 83.3 |
| Larsen et al[65], 2009 | 365 | Histology, follow up CT | - | 80 | - | 98.0 |
| Piscaglia et al[66], 2007 | 107 | CT, FNA, follow up | 0.77 | 0.95 | - | - |
| Konopke et al[57], 2007 | 100 | IOUS | 0.56 | 0.84 | 0.93 | 0.84 |
| Larsen et al[67], 2007 | 365 | FNA, CT, IOUS | 0.69 | 0.80 | 0.98 | 0.98 |
| Janica et al[68], 2007 | 51 | CT, FNA, follow up | 0.63 | 0.90 | - | - |
| Dietrich et al[69], 2006 | 131 | CT, MRI, FNA, follow up | 0.81 | 0.91 | - | - |
| Quaia et al[70], 2006 | 253 | FNA, CT, MRI, IOUS | 0.40 | 0.83 | 0.63 | 0.84 |
| Konopke et al[71], 2005 | 56 | IOUS, FNA, CT | 0.53 | 0.86 | 0.89 | 0.89 |
| Oldenburg et al[72], 2005 | 40 | CT, MRI | 0.69 | 0.90 | - | - |
| Albrecht et al[73], 2003 | 123 | CT, MRI, IOUS, FNA | 0.94 | 0.98 | 0.60 | 0.88 |
| Solbiati et al[11], 2001 | 32 | CT | - | 21 out of 32, 94 more metastases than US | - | - |
| Bernatik et al[58], 2001 | 28 | CT | 0.59 | 0.97 | - | - |
| Albrecht et al[74], 2001 | 62 | CT, MRI, IOUS, FNA | 0.92 | 0.97 | - | - |
IOUS: Intra operative ultrasound; SPIO-MRI: Superparamagnetic iron oxide-enhanced magnetic resonance imaging; Gd-EOB-MRI: Gadoxetic acid-enhanced magnetic resonance imaging; CT: Computed tomography; FNA: Fine needle aspiration; US: Ultrasound; CEUS: Contrast enhanced ultrasound.
Figure 1.
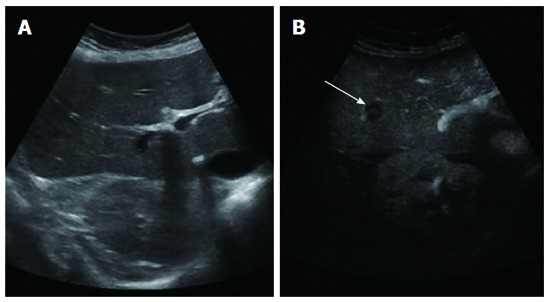
Baseline ultrasound did not detect any lesion (A), while contrast enhanced ultrasound clearly showed one hypoenhancing lesion (B) (arrow).
However, it is noteworthy that most of the studies have used imaging modalities, mostly CT examinations and sometimes CT, MRI, and intra-operative US, as standards of reference and for follow up, and very few reports have included histologic or pathologic confirmation. Nevertheless, as CT and MRI are the modalities of choice for CRLM detection, comparison of CEUS with these techniques seems reasonable for practical purposes in evaluating the efficacy of CEUS (Figure 2). On the other hand, in clinical studies, histologic confirmation is sometimes difficult due to ethical limitations.
Figure 2.
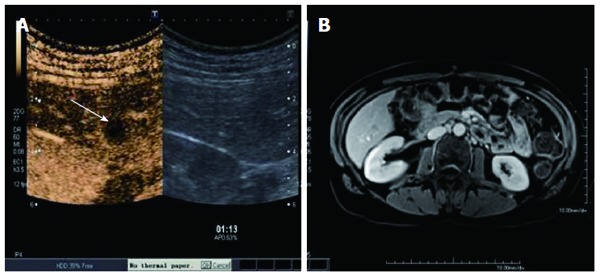
Both contrast enhanced ultrasound (A), (arrow showing the metastases) and BOPTA-enhanced magnetic resonance imaging detected a liver metastasis (B).
An early study by Bernatik et al[58] in 2001 investigated the diagnostic yield of CEUS compared to helical CT in the detection of liver metastases without a histological diagnosis. They found that CEUS showed 97% of the lesions seen by CT. Table 1 summarizes the results of the relevant studies published from 2001 to July 2013. The first encouraging results were subsequently confirmed by a series of studies in the following years, during which time the improvements in US devices and the quality of imaging with lower MI and better contrast and resolution have continued. It is also noteworthy that CT and MRI technology has also improved during this period and now offer better diagnostic accuracy. In a clinical study, Piscaglia et al[50] examined 109 patients with colorectal cancer (n = 92 patients) and gastric cancer (n = 17 patients) with US, CEUS and CT. As the gold standard, CT, histopathology or follow up was used. CEUS improved sensitivity for the detection of liver metastases from 76.9%, as obtained by US, to 95.4%, whereas CT had a sensitivity of 90.8%. In 15 patients (13.8%), CEUS revealed more metastases than CT, while CT revealed more metastases than CEUS in 9 patients (8.2%). The authors concluded that “Findings at CEUS and CT appear to be complementary in achieving maximum sensitivity”.
In 2010, Cantisani et al[59] published a case series of 110 patients with suspected hepatic metastases from CRC and who were evaluated prospectively with US, CEUS and CT by two independent readers. The gold standard of reference was IOUS (n = 45) or a follow-up of at least 6 mo with MDCT or Gd-BOPTA-enhanced MRI. On a patient-by-patient analysis, CEUS improved US sensitivity from 67.4%-71.6% to 93.4%-95.8%. On a lesion-by-lesion analysis, CEUS improved the sensitivity of US from 60.9%-64.9% to 85.3%-92.8% and increased its specificity from 50%-60% to 76.7%-83.3%.
In 2010, Marinetti et al[46] studied 34 patients with 57 hepatic lesions and found that Gd- and SPIO-enhanced MRI was the most accurate modality for the identification of CRLM, while PET/CT was the most sensitive. Additionally, both of them performed better than CT and CEUS. The gold standard included surgical findings and MDCT follow-up. Muhi et al[60] compared the accuracy of contrast-enhanced CT, CEUS, superparamagnetic iron oxide-enhanced MRI (SPIO-MRI), and gadoxetic acid-enhanced MRI (Gd-EOB-MRI) for CRLM detection in 111 CRC patients. They found that Gd-EOB-MRI (sensitivity 95%) and SPIO-MRI were more accurate than CT and CEUS, particularly for lesions ≤ 1 cm, but that CEUS was more sensitive than CT (73% vs 63%).
In 2010, Cabassa et al[61] performed a detailed systematic review on published CEUS research for liver metastasis, which included an extensive search of the literature. Out of 14 eligible papers, only three articles fulfilled the quality assessment, and these comprised a total of 450 patients (patient sample number: range 12-365; cancer prevalence: 14.8%-71.2%). The estimated per-patient sensitivity ranged from 79%-100%, but a meta-analysis was not carried out because of the lack of eligible studies. The authors concluded that “CEUS seems to be promising in the detection of liver metastases; however, there have not been enough studies to conduct meta-analysis. Further studies are required before this promising method can be widely used”.
In 2013[19], a very detailed analysis of 19 selected eligible studies on liver CEUS with SonoVue that were judged to be of a low risk of bias was published. CEUS performed equally to liver CT and MRI in the surveillance of cirrhosis and the characterization of incidentally detected focal liver lesions but was more cost effective. For CRLM detection, CEUS had a similar cost and efficacy as CT but was superior to MRI. However, the authors also detailed numerous limitations of the analyzed studies and concluded that further research is required to verify the economic aspects and to accurately compare the efficacy of CEUS, CT and MRI, on therapeutic planning, treatment and clinical outcomes.
Intra-operative CEUS also seems to be a viable and successful technique, as shown in a report by Itabashi et al[62] in 2013. In that study, out of a total of 454 patients who were examined with intra-operative CEUS, CRLM was detected in an additional 71 patients compared to intra-operative US.
LIMITATIONS OF CEUS
Although CEUS may be considered an effective technique, as reported above, it still presents the same important drawbacks of every US examination, including operator dependency, and has limitations in obese patients, non-compliant subjects, and in cases with abundant meteorism or intestinal interposition. For these reasons, if the B-mode US is unsatisfactory, the subsequent CEUS examination will be suboptimal.
A specific limitation of CEUS in studying the liver is that usually only lesions larger than 3-5 mm are detectable. This is due to the limited spatial resolution of CEUS images[63] and, as such, very small metastases may be missed. The US study of the subdiaphragmatic liver by subcostal scanning is sometimes inadequate, especially in patients with a high lying diaphragm (eventratio diafragmatica), or in cases where there is the interposition of the intestine. To avoid missing metastases in these high locations, a meticulous operator is required. Specifically, during the temporal span of contrast presence in liver, the operator must switch to intercostal scanning and also reposition the patient onto the left decubitus. Liver steatosis, which is often induced by chemotherapy, can also be an important limitation in CEUS imaging, as its presence can increase the possibility of missing deep-seated metastases. In these cases, the operator must routinely try to bring the deeper parts of the liver closer to the transducer with left lateral decubitus positioning and intercostal scanning. As such, CEUS needs considerable operator expertise to avoid variations in results, and training is required. Additionally, CEUS may not accurately delineate the segmental localization of metastases and their 3D-shape, and for this reason, its utility in surgical assessment is limited. Even with the recent use of PACS, image documentation and review for CEUS examination still lags behind the exact documentation used in CT and MRI. As such, making accurate comparisons with old CEUS studies and following up suspected liver lesions can be more challenging with the current US and CEUS documentation.
ROLE OF CEUS IN THE MANAGEMENT OF CRC PATIENTS
An indisputable advantage of CEUS is its superior accuracy in comparison with US (Table 1). Accordingly, any US liver evaluation in a CRC patient with a known or suspected diagnosis should be a CEUS examination. The metastases missed by US are unacceptable in the management of the CRC patient, both in terms of success of treatment and of costs. The EFSUMB guidelines for CRLM detection, which were updated in 2012[15], state that the use of CEUS is recommended for the following indications: (1) To characterize indeterminate (usually small) lesions shown on either CT or MRI; (2) To “rule out” liver metastases or abscesses unless conventional ultrasound shows typical findings; (3) For treatment planning in selected cases to assess the number and location of liver metastases, either alone or complementarily with CT and/or MRI; and (4) Surveillance of oncology patients where CEUS has previously been useful. It was also recommended that unenhanced US should be replaced with CEUS for the evaluation of liver metastases in colorectal cancer patients after chemotherapy. Finally, the EFSUMB guidelines recommend the use of intra-operative liver CEUS in “the detection of liver metastases in all patients undergoing liver resection” and in “the targeting of occult lesions for ablation therapy for patients undergoing combined liver resection and ablative therapy”.
The immediateness of diagnosis that CEUS offers when a suspected liver lesion is revealed during a US examination is particularly notable. This is usually accompanied by patient satisfaction, as they do not need to make another appointment for a CT or MRI exam or suffer the anxiety of waiting in uncertainty.
However, for surgical planning, the comprehensive information offered by CT and MRI cannot be achieved with CEUS.
Furthermore, as reported by Bolondi[64], although at present the use of CEUS is largely accepted in clinical practice and the technique has been implemented in most centers for more than a decade, its exact allocation in the diagnostic algorithm has not yet been established. Many reasons may explain this. For example, most clinicians still consider CEUS as a supplement to CT and MRI, and they still prefer the latter because as with any ultrasound technique, it has several limitations, as reported above, and may not contribute as promptly to staging as CT.
CONCLUSION
According to the data present in Literature and as reported by EFSUMB Guidelines, CEUS should be the correct replacement of baseline US in every occasion where US is normally employed to follow patients suspected to develop CRLM. CEUS should also be a valuable alternative when a contrast study is needed and CT and MRI contrast are contraindicated, as in kidney failure patients, and also in case of inconclusive MRI/CT.
However, at times, the impossibility to study, with absolute certainty, the entire liver, and the inadequateness of the US to stage extra-liver colo-rectal metastases do not permit to recommend CEUS as a real alternative in colo-rectal patients preoperative staging but only, modifying the Bipat assertion, CEUS is highly efficient in helping to distinguish between two groups of patients with liver metastases: the group of patients with diffuse metastases who are no longer eligible for curative treatment and the group with no metastases or a very limited number of them. The patients in the latter group require CT, MR imaging, or FDG PET for the selection of appropriate therapeutic approaches.
Footnotes
P- Reviewer: Bramhall S, Chiu KW, Gruttadauria S, Hilmi IA, Lankarani KB, Morioka D S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Albrecht T, Hohmann J, Oldenburg A, Skrok J, Wolf KJ. Detection and characterisation of liver metastases. Eur Radiol. 2004;14 Suppl 8:P25–P33. doi: 10.1007/s10406-004-0088-z. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove DO, Bolondi L. Malignant liver disease. In: Cosgrove D, Meire H, Dewbury K, editors. Abdominal and general ultrasound. Edinburgh: Churchill Livingstone; 1993. pp. 271–293. [Google Scholar]
- 3.Glover C, Douse P, Kane P, Karani J, Meire H, Mohammadtaghi S, Allen-Mersh TG. Accuracy of investigations for asymptomatic colorectal liver metastases. Dis Colon Rectum. 2002;45:476–484. doi: 10.1007/s10350-004-6224-y. [DOI] [PubMed] [Google Scholar]
- 4.Xu LH, Cai SJ, Cai GX, Peng WJ. Imaging diagnosis of colorectal liver metastases. World J Gastroenterol. 2011;17:4654–4659. doi: 10.3748/wjg.v17.i42.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schima W, Kulinna C, Langenberger H, Ba-Ssalamah A. Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging. 2005;5 Spec No A:S149–S156. doi: 10.1102/1470-7330.2005.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Primrose JN. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 9.Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis--meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove D. Achieving optimal diagnostic yield through the use of real-time contrast enhanced ultrasonography (CEUS) Eur Radiol. 2007;17(Suppl 6):F71–F72. [Google Scholar]
- 11.Lencioni R, Della Pina C, Crocetti L, Bozzi E, Cioni D. Clinical management of focal liver lesions: the key role of real-time contrast-enhanced US. Eur Radiol. 2007;17 Suppl 6:F73–F79. doi: 10.1007/s10406-007-0231-8. [DOI] [PubMed] [Google Scholar]
- 12.Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver leasions. Eur Radiol. 2001;11 Suppl 3:E15–E26. doi: 10.1007/pl00014125. [DOI] [PubMed] [Google Scholar]
- 13.Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183–188. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmondson HA, Craig JR. Neoplasms of the liver. In: Schiff L, editor. Diseases of the liver. 8th ed. Philadelphia: Lippincott; 1987. [Google Scholar]
- 15.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 16.Furlan A, Marin D, Cabassa P, Taibbi A, Brunelli E, Agnello F, Lagalla R, Brancatelli G. Enhancement pattern of small hepatocellular carcinoma (HCC) at contrast-enhanced US (CEUS), MDCT, and MRI: intermodality agreement and comparison of diagnostic sensitivity between 2005 and 2010 American Association for the Study of Liver Diseases (AASLD) guidelines. Eur J Radiol. 2012;81:2099–2105. doi: 10.1016/j.ejrad.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Hohmann J, Albrecht T, Oldenburg A, Skrok J, Wolf KJ. Liver metastases in cancer: detection with contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:669–681. doi: 10.1007/s00261-004-0175-6. [DOI] [PubMed] [Google Scholar]
- 18.Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515–1526. doi: 10.1016/j.ultrasmedbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J, et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1–243. doi: 10.3310/hta17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica GT, Ji H, Ros PR. CT and MR imaging of hepatic metastases. AJR Am J Roentgenol. 2000;174:691–698. doi: 10.2214/ajr.174.3.1740691. [DOI] [PubMed] [Google Scholar]
- 21.Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89:757–762. doi: 10.1046/j.1365-2168.2002.02111.x. [DOI] [PubMed] [Google Scholar]
- 22.Valls C, Andía E, Sánchez A, Gumà A, Figueras J, Torras J, Serrano T. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218:55–60. doi: 10.1148/radiology.218.1.r01dc1155. [DOI] [PubMed] [Google Scholar]
- 23.Portugaller HR, Stacher R, Komaz G, Aschauer M, Hausegger KA, Szolar DH. [The value of different spiral CT phases in the detection of liver metastases] Rofo. 2002;174:452–458. doi: 10.1055/s-2002-25122. [DOI] [PubMed] [Google Scholar]
- 24.Rappeport ED, Loft A. Liver metastases from colorectal cancer: imaging with superparamagnetic iron oxide (SPIO)-enhanced MR imaging, computed tomography and positron emission tomography. Abdom Imaging. 2007;32:624–634. doi: 10.1007/s00261-007-9297-y. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Ko SW, Hwang SB, Kim CS, Yu HC. Detection and characterization of liver metastases: 16-slice multidetector computed tomography versus superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur Radiol. 2006;16:1337–1345. doi: 10.1007/s00330-005-0140-y. [DOI] [PubMed] [Google Scholar]
- 26.Scott DJ, Guthrie JA, Arnold P, Ward J, Atchley J, Wilson D, Robinson PJ. Dual phase helical CT versus portal venous phase CT for the detection of colorectal liver metastases: correlation with intra-operative sonography, surgical and pathological findings. Clin Radiol. 2001;56:235–242. doi: 10.1053/crad.2000.0668. [DOI] [PubMed] [Google Scholar]
- 27.Bipat S, Niekel MC, Comans EF, Nio CY, Bemelman WA, Verhoef C, Stoker J. Imaging modalities for the staging of patients with colorectal cancer. Neth J Med. 2012;70:26–34. [PubMed] [Google Scholar]
- 28.Bipat S, van Leeuwen MS, Ijzermans JN, Comans EF, Planting AS, Bossuyt PM, Greve JW, Stoker J. Evidence-base guideline on management of colorectal liver metastases in the Netherlands. Neth J Med. 2007;65:5–14. [PubMed] [Google Scholar]
- 29.Coenegrachts K, De Geeter F, ter Beek L, Walgraeve N, Bipat S, Stoker J, Rigauts H. Comparison of MRI (including SS SE-EPI and SPIO-enhanced MRI) and FDG-PET/CT for the detection of colorectal liver metastases. Eur Radiol. 2009;19:370–379. doi: 10.1007/s00330-008-1163-y. [DOI] [PubMed] [Google Scholar]
- 30.Coenegrachts K, Orlent H, ter Beek L, Haspeslagh M, Bipat S, Stoker J, Rigauts H. Improved focal liver lesion detection: comparison of single-shot spin-echo echo-planar and superparamagnetic iron oxide (SPIO)-enhanced MRI. J Magn Reson Imaging. 2008;27:117–124. doi: 10.1002/jmri.21247. [DOI] [PubMed] [Google Scholar]
- 31.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim KW, Byun JH, Won HJ, Shin YM, Kim PN, Lee MS, Lee MG. Comparison of mangafodipir trisodium- and ferucarbotran-enhanced MRI for detection and characterization of hepatic metastases in colorectal cancer patients. AJR Am J Roentgenol. 2006;186:1059–1066. doi: 10.2214/AJR.04.1941. [DOI] [PubMed] [Google Scholar]
- 33.Blyth S, Blakeborough A, Peterson M, Cameron IC, Majeed AW. Sensitivity of magnetic resonance imaging in the detection of colorectal liver metastases. Ann R Coll Surg Engl. 2008;90:25–28. doi: 10.1308/003588408X242303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones WE, Thomas CR, Herman JM, Abdel-Wahab M, Azad N, Blackstock W, Das P, Goodman KA, Hong TS, Jabbour SK, et al. ACR appropriateness criteria® resectable rectal cancer. Radiat Oncol. 2012;7:161. doi: 10.1186/1748-717X-7-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Erkel AR, Pijl ME, van den Berg-Huysmans AA, Wasser MN, van de Velde CJ, Bloem JL. Hepatic metastases in patients with colorectal cancer: relationship between size of metastases, standard of reference, and detection rates. Radiology. 2002;224:404–409. doi: 10.1148/radiol.2242011322. [DOI] [PubMed] [Google Scholar]
- 36.Suh WW, Blackstock AW, Herman J, Konski AA, Mohiuddin M, Poggi MM, Regine WF, Cosman BC, Saltz L, Johnstone PA. ACR Appropriateness Criteria on resectable rectal cancer: expert panel on radiation oncology--rectal/anal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1427–1430. doi: 10.1016/j.ijrobp.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Ward J, Robinson PJ, Guthrie JA, Downing S, Wilson D, Lodge JP, Prasad KR, Toogood GJ, Wyatt JI. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology. 2005;237:170–180. doi: 10.1148/radiol.2371041444. [DOI] [PubMed] [Google Scholar]
- 38.Kim MJ, Kim JH, Chung JJ, Park MS, Lim JS, Oh YT. Focal hepatic lesions: detection and characterization with combination gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2003;228:719–726. doi: 10.1148/radiol.2283020735. [DOI] [PubMed] [Google Scholar]
- 39.del Frate C, Bazzocchi M, Mortele KJ, Zuiani C, Londero V, Como G, Zanardi R, Ros PR. Detection of liver metastases: comparison of gadobenate dimeglumine-enhanced and ferumoxides-enhanced MR imaging examinations. Radiology. 2002;225:766–772. doi: 10.1148/radiol.2253011854. [DOI] [PubMed] [Google Scholar]
- 40.Soyer P, Poccard M, Boudiaf M, Abitbol M, Hamzi L, Panis Y, Valleur P, Rymer R. Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology. 2004;231:413–420. doi: 10.1148/radiol.2312021639. [DOI] [PubMed] [Google Scholar]
- 41.Kim YK, Lee JM, Kim CS, Chung GH, Kim CY, Kim IH. Detection of liver metastases: gadobenate dimeglumine-enhanced three-dimensional dynamic phases and one-hour delayed phase MR imaging versus superparamagnetic iron oxide-enhanced MR imaging. Eur Radiol. 2005;15:220–228. doi: 10.1007/s00330-004-2570-3. [DOI] [PubMed] [Google Scholar]
- 42.Nasu K, Kuroki Y, Nawano S, Kuroki S, Tsukamoto T, Yamamoto S, Motoori K, Ueda T. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–130. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 43.Szurowska E, Nowicki TK, Izycka-Swieszewska E, Zadrozny D, Markiet K, Studniarek M. Predictive value of apparent diffusion coefficient in evaluation of colorectal carcinoma hepatic metastases’ response to radiofrequency ablation. J Magn Reson Imaging. 2013;38:1027–1032. doi: 10.1002/jmri.24089. [DOI] [PubMed] [Google Scholar]
- 44.Lambregts DM, Maas M, Cappendijk VC, Prompers LM, Mottaghy FM, Beets GL, Beets-Tan RG. Whole-body diffusion-weighted magnetic resonance imaging: current evidence in oncology and potential role in colorectal cancer staging. Eur J Cancer. 2011;47:2107–2116. doi: 10.1016/j.ejca.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Frankel TL, Gian RK, Jarnagin WR. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J Gastrointest Oncol. 2012;3:11–18. doi: 10.3978/j.issn.2078-6891.2012.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, Tanga M, Persico F, Addeo P, D’Antonio D, et al. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging. 2010;35:511–521. doi: 10.1007/s00261-009-9555-2. [DOI] [PubMed] [Google Scholar]
- 47.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 48.Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol. 2011;46:548–555. doi: 10.1097/RLI.0b013e31821a2163. [DOI] [PubMed] [Google Scholar]
- 49.Dawson P, Cosgrove D, Grainger R. Textbook of contrast media. Oxford: ISIS Medical Media; 1999. [Google Scholar]
- 50.Piscaglia F, Lencioni R, Sagrini E, Pina CD, Cioni D, Vidili G, Bolondi L. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med Biol. 2010;36:531–550. doi: 10.1016/j.ultrasmedbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Albrecht T. Detection and characterisation of liver metastases. In: Lencioni R, editor. Enhancing the role of ultrasound with contrast agents. Pisa: Springer-Verlag Italia; 2006. pp. 53–67. [Google Scholar]
- 52.Larsen LP. Role of contrast enhanced ultrasonography in the assessment of hepatic metastases: A review. World J Hepatol. 2010;2:8–15. doi: 10.4254/wjh.v2.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreft B, Pauleit D, Bachmann R, Conrad R, Krämer A, Schild HH. [Incidence and significance of small focal liver lesions in MRI] Rofo. 2001;173:424–429. doi: 10.1055/s-2001-13340. [DOI] [PubMed] [Google Scholar]
- 54.Jones EC, Chezmar JL, Nelson RC, Bernardino ME. The frequency and significance of small (less than or equal to 15 mm) hepatic lesions detected by CT. AJR Am J Roentgenol. 1992;158:535–539. doi: 10.2214/ajr.158.3.1738990. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz LH, Gandras EJ, Colangelo SM, Ercolani MC, Panicek DM. Prevalence and importance of small hepatic lesions found at CT in patients with cancer. Radiology. 1999;210:71–74. doi: 10.1148/radiology.210.1.r99ja0371. [DOI] [PubMed] [Google Scholar]
- 56.Cosgrove D, Blomley M. Liver tumors: evaluation with contrast-enhanced ultrasound. Abdom Imaging. 2004;29:446–454. doi: 10.1007/s00261-003-0126-7. [DOI] [PubMed] [Google Scholar]
- 57.Konopke R, Kersting S, Bergert H, Bloomenthal A, Gastmeier J, Saeger HD, Bunk A. Contrast-enhanced ultrasonography to detect liver metastases: a prospective trial to compare transcutaneous unenhanced and contrast-enhanced ultrasonography in patients undergoing laparotomy. Int J Colorectal Dis. 2007;22:201–207. doi: 10.1007/s00384-006-0134-5. [DOI] [PubMed] [Google Scholar]
- 58.Bernatik T, Strobel D, Hahn EG, Becker D. Detection of liver metastases: comparison of contrast-enhanced wide-band harmonic imaging with conventional ultrasonography. J Ultrasound Med. 2001;20:509–515. doi: 10.7863/jum.2001.20.5.509. [DOI] [PubMed] [Google Scholar]
- 59.Cantisani V, Ricci P, Erturk M, Pagliara E, Drudi F, Calliada F, Mortele K, D’Ambrosio U, Marigliano C, Catalano C, et al. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31:500–505. doi: 10.1055/s-0028-1109751. [DOI] [PubMed] [Google Scholar]
- 60.Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, Sano K, Sano M, Kato S, Kitamura T, Fatima Z, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34:326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- 61.Cabassa P, Bipat S, Longaretti L, Morone M, Maroldi R. Liver metastases: Sulphur hexafluoride-enhanced ultrasonography for lesion detection: a systematic review. Ultrasound Med Biol. 2010;36:1561–1567. doi: 10.1016/j.ultrasmedbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 62.Itabashi T, Sasaki A, Otsuka K, Kimura T, Nitta H, Wakabayashi G. Potential value of sonazoid-enhanced intraoperative laparoscopic ultrasonography for liver assessment during laparoscopy-assisted colectomy. Surg Today. 2014;44:696–701. doi: 10.1007/s00595-013-0607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leoni S, Piscaglia F, Golfieri R, Camaggi V, Vidili G, Pini P, Bolondi L. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol. 2010;105:599–609. doi: 10.1038/ajg.2009.654. [DOI] [PubMed] [Google Scholar]
- 64.Bolondi L. The appropriate allocation of CEUS in the diagnostic algorithm of liver lesions: a debated issue. Ultrasound Med Biol. 2013;39:183–185. doi: 10.1016/j.ultrasmedbio.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Larsen LP, Rosenkilde M, Christensen H, Bang N, Bolvig L, Christiansen T, Laurberg S. Can contrast-enhanced ultrasonography replace multidetector-computed tomography in the detection of liver metastases from colorectal cancer? Eur J Radiol. 2009;69:308–313. doi: 10.1016/j.ejrad.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Piscaglia F, Corradi F, Mancini M, Giangregorio F, Tamberi S, Ugolini G, Cola B, Bazzocchi A, Righini R, Pini P, et al. Real time contrast enhanced ultrasonography in detection of liver metastases from gastrointestinal cancer. BMC Cancer. 2007;7:171. doi: 10.1186/1471-2407-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsen LP, Rosenkilde M, Christensen H, Bang N, Bolvig L, Christiansen T, Laurberg S. The value of contrast enhanced ultrasonography in detection of liver metastases from colorectal cancer: a prospective double-blinded study. Eur J Radiol. 2007;62:302–307. doi: 10.1016/j.ejrad.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Janica JR, Lebkowska U, Ustymowicz A, Augustynowicz A, Kamocki Z, Werel D, Polaków J, Kedra B, Pepinski W. Contrast-enhanced ultrasonography in diagnosing liver metastases. Med Sci Monit. 2007;13 Suppl 1:111–115. [PubMed] [Google Scholar]
- 69.Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, Vossas U, Hauenstein K, Koch W, Blank W, et al. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol. 2006;12:1699–1705. doi: 10.3748/wjg.v12.i11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quaia E, D’Onofrio M, Palumbo A, Rossi S, Bruni S, Cova M. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidence. Eur Radiol. 2006;16:1599–1609. doi: 10.1007/s00330-006-0192-7. [DOI] [PubMed] [Google Scholar]
- 71.Konopke R, Kersting S, Saeger HD, Bunk A. [Detection of liver lesions by contrast-enhanced ultrasound -- comparison to intraoperative findings] Ultraschall Med. 2005;26:107–113. doi: 10.1055/s-2005-858095. [DOI] [PubMed] [Google Scholar]
- 72.Oldenburg A, Hohmann J, Foert E, Skrok J, Hoffmann CW, Frericks B, Wolf KJ, Albrecht T. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med. 2005;26:277–284. doi: 10.1055/s-2005-858526. [DOI] [PubMed] [Google Scholar]
- 73.Albrecht T, Oldenburg A, Hohmann J, Skrok J, Hoffmann CW, Schettler S, Wolf KJ. Imaging of liver metastases with contrast-specific low-MI real-time ultrasound and SonoVue. Eur Radiol. 2003;13 Suppl 3:N79–N86. doi: 10.1007/s00330-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 74.Albrecht T, Hoffmann CW, Schmitz SA, Schettler S, Overberg A, Germer CT, Wolf KJ. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol. 2001;176:1191–1198. doi: 10.2214/ajr.176.5.1761191. [DOI] [PubMed] [Google Scholar]


