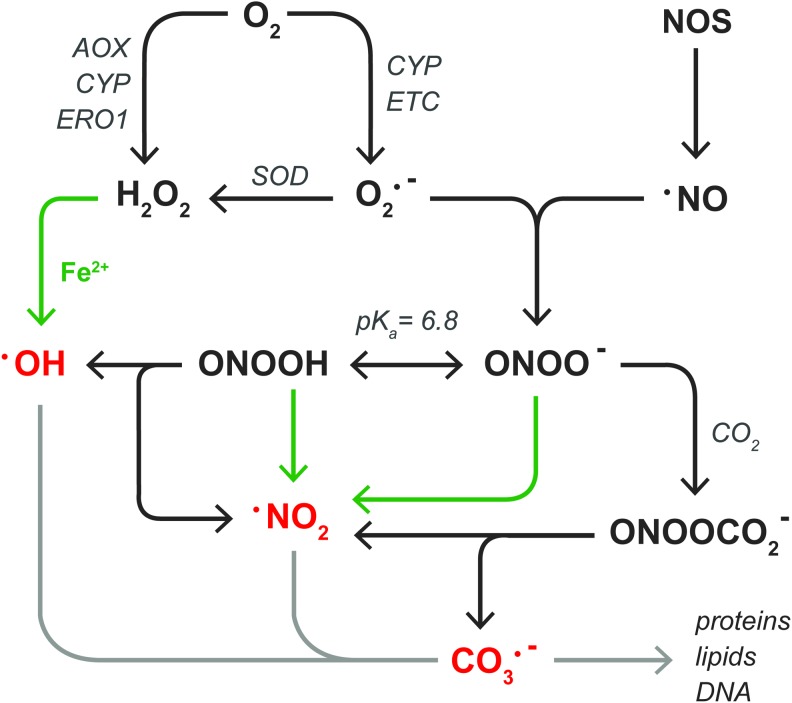
FIG. 3.
ROS and RNS relevant to NAFLD and hepatic IR. Superoxide anion (O2•−) is formed in the one-electron reduction of O2 by CYP enzymes or the ETC. Thereafter, different SODs catalyze the dismutation of O2•− into hydrogen peroxide (H2O2). In addition, H2O2 is formed in the two-electron reduction of O2 by AOX, CYP enzymes, or ERO1. H2O2 can react with transition metals such as ferrous iron (Fe2+, green arrows) to form hydroxyl radical (•OH). Alternatively, O2•− reacts with NOS-derived nitric oxide (•NO) to form peroxynitrite anion (ONOO−), which exists in equilibrium with its conjugate acid peroxynitrous acid (ONOOH, pKa=6.8). Both forms of peroxynitrite can react with Fe2+ to generate nitrogen dioxide (•NO2). •NO2 is also formed during the homolytic fission of ONOOH, along with •OH, as well as in the reaction between ONOO- and CO2, also yielding carbonate radical anion (CO3•−). The free radicals •OH, •NO2, and CO3•− are indicated in red to emphasize their high reactivity, which enables them to irreversibly alter the chemical structure of biomolecules (light gray arrow) as described in section “Molecular targets of ROS/RNS in NAFLD and IR.” AOX, fatty acyl-CoA oxidase; ERO1, ER oxidoreductin 1; ETC, electron transport chain; IR, ischemia-reperfusion; NOS, nitric oxide synthase; SOD, superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars