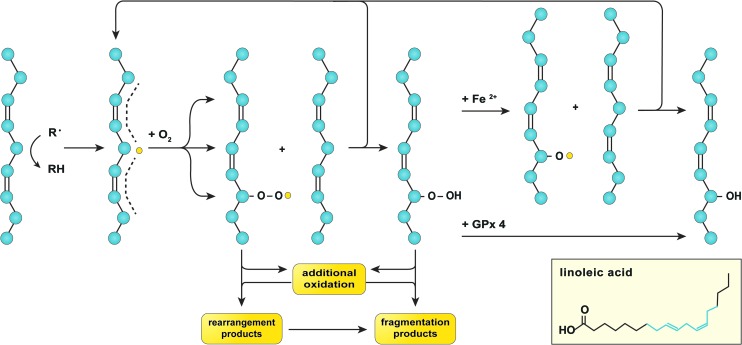
FIG. 4.
Lipid peroxidation. Lipid peroxidation is initiated by hydrogen abstraction, mostly from hydrocarbons that are flanked by two alkenes (depicted on the left), that is, those on the aliphatic chains of PUFAs such as linoleic acid (inset, in which the structure highlighted in blue corresponds to the aliphatic structure in the figure). The formed carbon-centered lipid radical (L•, yellow dot) has the ability to relocate three carbon atoms away from the abstraction site (dashed line), where it swiftly reacts with oxygen to form a lipid peroxyl radical (L–OO•). Subsequently, L–OO• can undergo intramolecular modification to form rearrangement products, additional oxidation, or react with a proximal PUFA to generate a lipid hydroperoxide (L–OOH) as well as a new L• (top arrow). L–OOH can undergo additional oxidation or dissociate into fragmentation products. Alternatively, L–OOH can react with GPx 4 to form a lipid hydroxide (L–OH) or undergo ferrous iron (Fe2+)-catalyzed oxidation to form a lipid alkoxyl radical (L–O•), the latter of which has the ability to oxidize another PUFA and generate a new L• (top arrow) as well as a L–OH. All these events lead to membrane destabilization as a result of lipid packing defects, which may have detrimental consequences on cell function and viability. GPx 4, glutathione peroxidase 4; PUFA, polyunsaturated fatty acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars