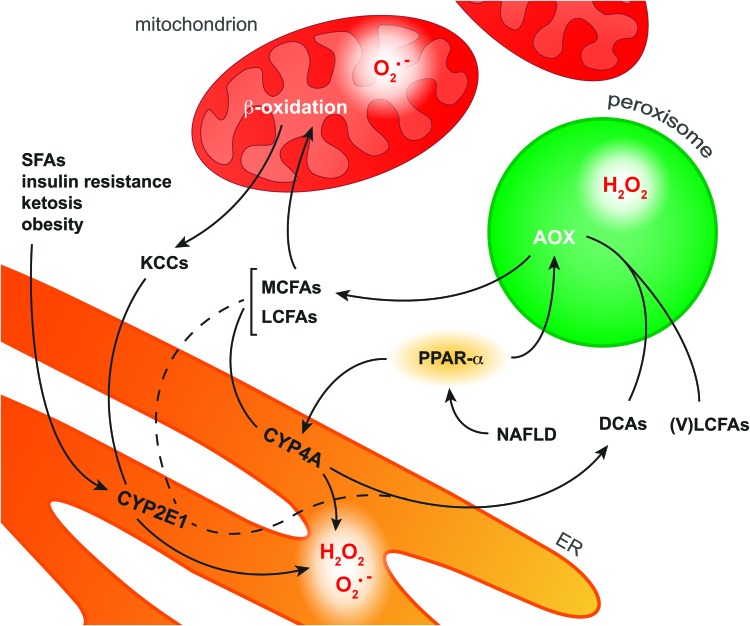
FIG. 9.
Pathways of FA oxidation in hepatocytes. In NAFLD, activation of the transcription factor PPAR-α induces upregulation of the enzymes AOX and CYP4A in peroxisomes and the ER, respectively. CYP4A catalyzes ω-oxidation of MCFAs and LCFAs as a result of which DCAs as well as O2•− and H2O2 are formed. AOX is the first enzyme in the peroxisomal β-oxidation system that converts VLCFAs and ER-derived DCAs into MCFAs, during which H2O2 is formed. MCFAs and LCFAs are transported into the mitochondria to undergo β-oxidation. However, when the mitochondrial β-oxidation system is overwhelmed with substrate, O2•− is formed along with KCCs that diffuse into the cytosol. Cytosolic KCCs are metabolized into glucose by CYP2E1, which is induced in response to various NAFLD-related stimuli such as increased levels of SFAs. In addition, CYP2E1 selectively catalyzes ω-oxidation of the FAs arachidonic acid and lauric acid (dashed line), producing O2•− and H2O2 as byproducts. The ROS produced during these processes can form secondary and tertiary ROS/RNS derivatives (section “ROS/RNS and Their Chemical Properties in the Context of NAFLD and IR”) that are capable of oxidizing biomolecules (section “Molecular Targets of ROS/RNS in NAFLD and IR”). DCA, dicarboxylic acid; KCC, ketone-containing catabolite; (V)LCFA, (very) long-chain fatty acid; MCFA, medium-chain fatty acid; PPAR-α, peroxisome proliferator-activated receptor α; SFA, saturated fatty acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars