Figure 6.
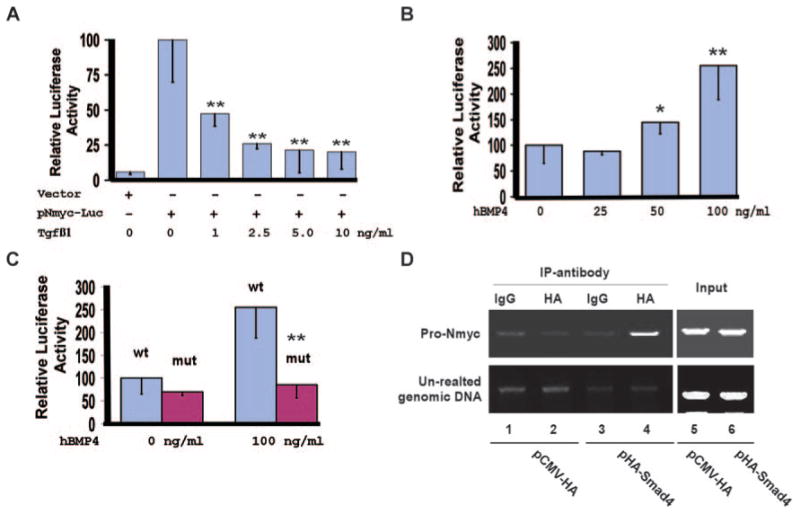
Nmyc is a direct downstream target of Smad4. A, NIH3T3 cells were transiently transfected with the reporter vector (pGL-basic) or pNmyc-luc, in which expression of luciferase is driven by the 351-bp Nmyc promoter. Cells were treated with 0 to 10 ng/mL of TGFβ1 for 48 hours, and luciferase activities were measured. The luciferase activity of pNmyc-luc without TGFβ1 stimulation was arbitrarily defined as 100 units. Data were averaged from 3 to 6 independent cultures, with error bars indicating SD. B, P19 cells were transiently transfected with pNmyc-Luc and were treated with 0 to 100 ng/mL hBMP4 for 48 hours. Luciferase analysis was then performed. The activity of the cell cultures without hBMP4 stimulation was defined as 100units. Data were averaged from 6 to 9 independent cultures. C, P19 cells were transfected with pNmyc-luc (wt) or pNmyc-luc-mut (mut), in which the potential Smad4-binding site within the Nmyc promoter was disrupted. Cells were treated with 0 or 100 ng/mL of hBMP4 for 48 hours, and luciferase activities were measured. BMP4 failed to upregulate expression of the reporter driven by the mutant Nmyc promoter. D, P19 cells were transiently transfected with pCMV-HA vector (negative control) or pHA-Smad4, which expresses HA-Smad4. ChIP analysis was performed with a rabbit IgG or an HA polyclonal antibody. HA-Smad4 formed a complex with the 351-bp Nmyc promoter but not with an unrelated genomic DNA sequence. The complex could be captured only by an anti-HA antibody and not by rabbit IgG. *P<0.1, **P<0.05.
