Figure 6.
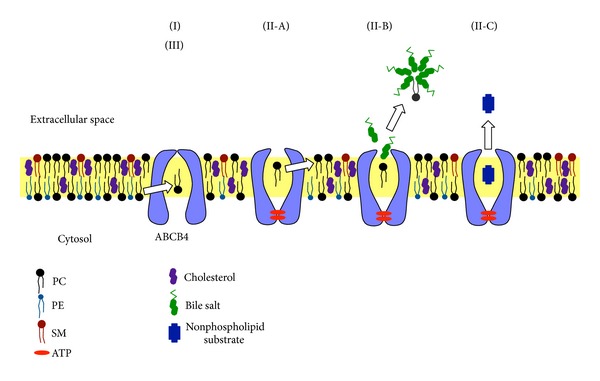
Model of molecular mechanism of ABCB4-mediated transport. (I) A substrate, mainly PC, enters the binding pocket of ABCB4 from the inner leaflet through the gaps between TMHs. (II) Binding and/or hydrolysis of ATP trigger a conformational change opening the binding pocket to the outer leaflet and the extracellular space. (II-A) In the absence of bile salts, a PC molecule laterally diffuses from the binding pocket of ABCB4 to the outer leaflet through the gaps between TMHs, which represents a floppase function of ABCB4. (II-B) In the presence of bile salts, a PC molecule is taken up from the binding pocket of ABCB4 by bile salt monomers, which represents an exporter function of ABCB4, and then a mixed bile salt/PC micelle is formed in the extracellular space. (II-C) A substrate with sufficient aqueous solubility directly diffuses from the binding pocket into the extracellular space regardless of the presence or absence of bile salts. (III) After dissociation of the substrate and ADP molecules, ABCB4 reset to the inward-facing state.
