Table 3.
Anti-proliferative activity of NA-2 derivatives with cyclic N-substitutions
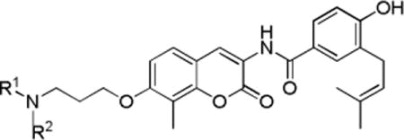
| ||||
|---|---|---|---|---|
| R1 | R2 | SKBr3 (μM) | MCF-7 (μM) | |
| 37a | Cyclohexyl | H | 0.66±0.12a | 1.07±0.35 |
| 37b | Cyclohexyl | CH3 | 0.97±0.03 | 1.08±0.07 |
| 37c | Phenyl | H | >50 | >50 |
| 37d | Phenyl | CH3 | >50 | >50 |
| 37e | Benzyl | H | 0.47±0.16 | 0.91±0.01 |
| 37f | (R)-1-phenylethyl | H | 1.00±0.31 | 1.45±0.20 |
| 37g | Cumenyl | H | 0.96±0.01 | 1.07±0.01 |
| 37h | Benzyl | CH3 | 10.73±0.49 | 11.52±4.16 |
| 37i |
|
H | 0.77±0.13 | 1.03±0.12 |
| 37j | Adamantyl | H | 0.31±0.04 | 0.32±0.03 |
Values represent mean ± standard deviation for at least two separate experiments performed in triplicate
