Figure 3.
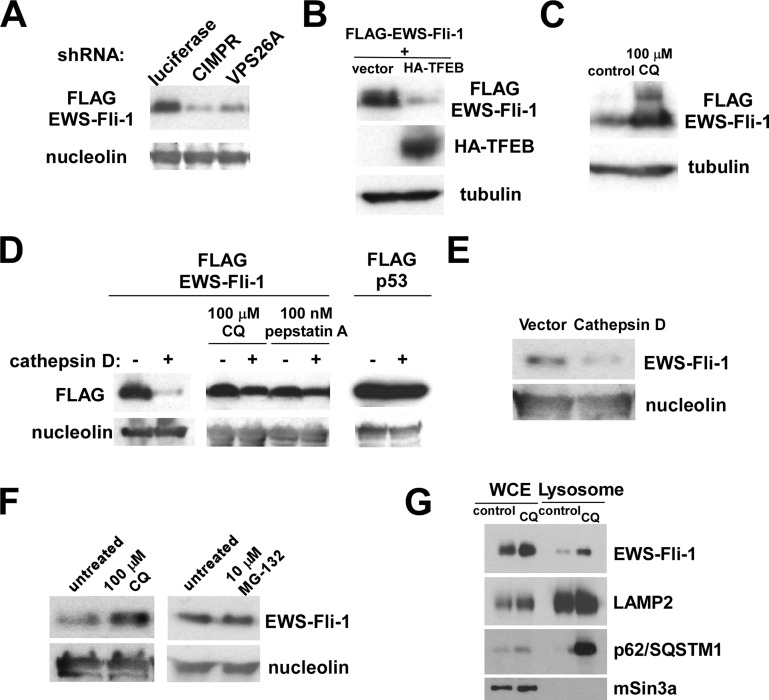
EWS-Fli-1 turns over by a lysosome-dependent mechanism: (A) Knockdown of CIMPR or VPS26A results in destabilization of FLAG-EWS-Fli-1. 293 cells were cotransfected with FLAG-EWS-Fli-1 and shRNA against luciferase (control), CIMPR, or VPS26A. Forty-eight hours after transfection, the levels of FLAG-EWS-Fli-1 were examined by anti-FLAG immunoblotting. Nucleolin serves as a loading control. (B) TFEB induces EWS-Fli-1 degradation in 293 cells. 293 cells were cotransfected with FLAG-EWS-Fli-1 and HA-TFEB or empty vector. Forty-eight hours after transfection, the levels of FLAG-EWS-Fli-1 were examined by anti-FLAG immunoblotting. Tubulin serves as a loading control. (C) Chloroquine stabilizes EWS-Fli-1 in 293 cells. 293 cells were transfected with FLAG-EWS-Fli-1. Transfected cells were left untreated (control) or treated with 100 μM chloroquine for 12 h. The levels of FLAG-EWS-Fli-1 were examined by anti-FLAG immunoblotting. Tubulin serves as a loading control. (D) Cathepsin D degrades EWS-Fli-1, but not p53, in 293 cells. 293 cells were cotransfected with FLAG-EWS-Fli-1 and cathepsin D or empty vector. Transfected cells were left untreated or treated with 100 μM chloroquine for 12 h or 100 nM pepstatin A for 12 h. 293 cells were cotransfected with FLAG-p53 and cathepsin D or empty vector. The levels of FLAG-EWS-Fli-1 and FLAG-p53 were examined by anti-FLAG immunoblotting. Nucleolin serves as a loading control. (E) Cathepsin D degrades endogenous EWS-Fli-1 in A673 Ewing sarcoma cells. A673 cells were infected with a lentivirus vector expressing cathepsin D or an empty vector, the infected cells were selected with puromycin, and the levels of endogenous EWS-Fli-1 were examined by anti-Fli-1 C-terminus antibody immunoblotting at 4 days after infection. Nucleolin serves as a loading control. (F) Chloroquine stabilizes endogenous EWS-Fli-1 in A673 cells. A673 cells were left untreated, treated with 100 μM chloroquine for 12 h, or treated with 10 μM MG-132 for 12 h. The levels of EWS-Fli-1 were examined by anti-Fli-1 C-terminus immunoblotting. While chloroquine increased the levels of endogenous EWS-Fli-1, MG-132 had no effect on the EWS-Fli-1 protein levels, suggesting that EWS-Fli-1 turns over by a lysosomal, but not proteasomal mechanism. (G) Endogenous EWS-Fli-1 in A673 cells displays increased lysosomal location upon chloroquine treatment. A673 cells were treated with 100 μM chloroquine for 12 h or left untreated, and the whole cell extract (WCE) and lysosomal fraction were isolated. The abundance of EWS-Fli-1, LAMP2, p62/SQSTM1, and mSin3A in each fraction was determined by immunoblotting.