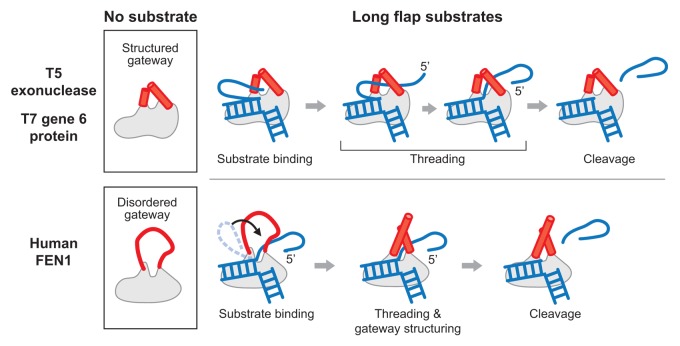
Figure 1. Mechanisms of cleavage of 5′-overhangs (flaps) by bacteriophage and human homologs. (Upper panel) Phage T5 exonuclease has a structured gateway domain region on the protein in the absence of DNA substrate. The predicted structure of T7 gp6 shows high similarity to the known structure of T5 exonuclease. The 5′-flap must be threaded through the gateway from the 5′-terminus until the scissile phosphodiester bond is positioned at the active site. A longer 5′-flap would require additional time to complete the threading process. (Lower panel) In human FEN1, the gateway domain region is disordered in the absence of substrate. Thus the aperture is considerably larger than that found in the structured gateway, allowing for more efficient passage of a long flap. The gateway then becomes structured after binding the substrate with the scissile phosphodiester bond in the active site. The disordered gateway is indicated as a red line and the structured gateway as two red cylinders. The DNA is shown in blue.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.