Abstract
Enterococcus, a Gram-positive facultative anaerobic cocci belonging to the lactic acid bacteria of the phylum Firmicutes, is known to be able to resist a wide range of hostile conditions such as different pH levels, high concentration of NaCl (6.5%), and the extended temperatures between 5°C and 65°C. Despite being the third most common nosocomial pathogen, our understanding on its virulence factors is still poorly understood. The current study was aimed to determine the prevalence of different virulence genes in Enterococcus faecalis and Enterococcus faecium. For this purpose, 79 clinical isolates of Malaysian enterococci were evaluated for the presence of virulence genes. pilB, fms8, efaAfm, and sgrA genes are prevalent in all clinical isolates. In conclusion, the pathogenicity of E. faecalis and E. faecium could be associated with different virulence factors and these genes are widely distributed among the enterococcal species.
1. Introduction
Enterococci are considered to be part of normal gut microbiota in both humans and animals and capable to survive in a diverse range of harsh conditions. Among them only two, that is, Enterococcus faecium and Enterococcus faecalis, are now being increasingly recognized to be involved in human infections such as bacteremia, endocarditis, urinary tract infections, and surgical site infections [1, 2]. This could be explained by their inherent resistance to various antibiotics with greater adaptability in hospital environments and has high genetic diversity as well as the presence of various virulence factors [3–5].
These opportunistic bacteria possess various virulence factors including enterococcal surface protein (Esp) and aggregation substance (Agg) which could enhance the colonization process in the host and binding to the host epithelium, respectively [6]. Others such as cytolysin [7], enterolysin A [8], gelatinase [9], hyaluronidase, Zn-metalloendopeptidase, enhanced expression of pheromone (Eep) [10], and adhesion-associated protein EfaA (E. faecalis endocarditis antigen A) [11] have been reported to be among the most important virulence factors.
Surprisingly through NCBI database analysis, there are some similarities observed in terms of their virulence determinants among enterococci. For instance, pilA in E. faecium has 99% similarity with the cell wall surface anchor family protein of Enterococcus sp. GMD4E (Accession: EKA01662). The pilB of E. faecium has 74% similarity with the cell wall surface anchor protein of E. faecalis B318 (Accession: ETU21897). Meanwhile, the ecbA in E. faecium has 92% similarity with the cell wall surface anchor protein of E. faecalis (Accession: WP_010708790) and 91% with Cna protein B-type domain protein E. faecalis (Accession: WP_002417581) (these regions are part of a collagen binding MSCRAMM, ecbA).
The cell wall adhesion (efaAfm) in E. faecium has 66% similarity with endocarditis specific antigen of E. faecalis T8 (Accession: EEU25576). With regard to the collagen binding MSCRAMM Acm (fms8) in E. faecium, 57% similarity has been observed with the collagen binding domain protein of E. faecalis (Accession: WP_016632409). The blast analysis for the collagen binding MSCRAMM scm in E. faecium has revealed 99% similarity with the collagen-binding MSCRAMM scm (Fms10) in Enterococcus sp. GMD5E. Finally, 37% similarity of the cell wall anchored protein sgrA in E. faecium has been detected when compared to the surface adhesion protein in Enterococcus sp. C1 (Accession: WP_008378446). These findings could be related to the notion that horizontal transfer of both resistance and virulence determinants is very common among enterococci [12]. Thus, this study was conducted to investigate the distribution of the diverse virulence factors and specify the dominant virulence genes among E. faecalis and E. faecium clinical isolates. Based on the similarity results and also the possibility of horizontal virulence gene transfer the pilA, pilB, hyl, ecbA, scm, fms8, efaAfm, and sgrA genes were chosen for this study.
2. Material and Methods
2.1. Bacterial Isolates
A total of 79 clinical isolates of E. faecalis (50 isolates) and E. faecium (29 isolates) were identified during May 2009 to March 2010 from a tertiary teaching hospital. Nonrepetitive isolates were collected from these samples such as urine, blood, pus, vaginal, and sterile body fluid.
2.2. DNA Extraction of Enterococcus
The DNA wasextracted using the DNA extraction kit (Gene ALL, South Korea) according to the manufacturer's instructions.
2.3. Evaluation of Different Virulence Genes
All isolates were subjected to the amplified pilA, pilB, hyl, ecbA, scm, fms8, efaAfm, and sgrA genes, using specific primers as listed in Table 1. These virulence genes are most strongly associated with clinical lineages of E. faecium; however, not much data has been reported among E. faecalis. The PCR amplification was carried out in a DNA thermocycler (Bio-Rad) using the amplification parameters as follows: initial denaturation at 95°C for 2 minutes, followed by 35 cycles of denaturation at 95°C for 20 seconds, annealing at 58°C for 10 seconds, and extension at 72°C for 20 seconds, with a final extension at 72°C for 5 minutes. All PCR products were analyzed by 1% agarose gel electrophoresis.
Table 1.
Characteristics of different primers used in the current study.
| Primer name | Sequence (5′-3′) | Size (bp) | Accession number |
|---|---|---|---|
| pilA | Forward: AAAACGCCACCAGAGAAGGT Reverse: CATTGGCGCAATCACAACCA |
459 | ENA∣ACI49671 |
|
| |||
| pilB | Forward: GATACCCAGCTGACGGCTTT Reverse: GGTACTGCCGAAAACGAAGC |
959 | EFF34880 |
|
| |||
| fms8 | Forward: AGACGAGCAGATGAACAGCC Reverse: CCCGTCAATCGTCGTACTGT |
765 | YP_006376887 |
|
| |||
| efaAfm | Forward: AAAAGGCAAGCGACGCAGAT Reverse: AGGTCTAGCCAAGCATGAGG |
199 | FJ609170 |
|
| |||
| sgrA | Forward: CTGATCGGATTGTTTATGA Reverse: AATAAACTTCCCCAATAACTT |
150 | AGS75503 |
|
| |||
| ecbA | Forward: GGAGTGAGGCTTTTAAACCAGA Reverse: GGAAACAGGGTACTTTG |
182 | AGS75851 |
|
| |||
| hyl | Forward: CCCTGGACACATGAAATGCG Reverse: AGCATCGGCCGTTGATAGAC |
605 | AF544400 |
|
| |||
| scm | Forward: GTTTACTAGTCCTAGTTGC Reverse: TCTGTACTGTCGCTTGTGTC |
1015 | YP_006377279 |
2.4. Purification of Virulence Genes from Agarose Gel
PCR products of each virulence gene were run on 1% (w/v) molecular grade agarose gel (Sigma, USA), using a Bio-Rad mini-gel electrophoresis system at 80 V for 70 minutes. The DNA was purified using DNA purification kit (Gene ALL, South Korea) according to the manufacturer's instructions.
2.5. Sequence Analysis
The PCR products were purified from gel agarose and then the purified products were sequenced by Sigma Company (Singapore). The results of DNA sequencing were run in Chromas Lite program to analyze the similarity to the sequenced gene in GenBank library.
3. Results and Discussion
A total of 79 E. faecalis (50 isolates) and E. faecium (29 isolates) were analyzed for the presence of different virulence genes including pilA, pilB, hyl, ecbA, scm, fms8, efaAfm, and sgrA. The analysis showed different prevalence of virulence genes in Enterococcus which ranged from 35.4% to 100%. The pilB, fms8, efaAfm, and sgrA were identified as the dominant virulence genes in all isolates. The second most prevalent virulence gene, scm, was found in 92.4% of the isolates (n = 73/79). The ecbA was determined as the third most common prevalent virulence gene with 81% (n = 64/79) frequency. The pilA showed 73.4% (n = 58/79) prevalence in clinical isolates of Enterococcus. The lowest prevalence detected was hyl with the prevalence of 35.4% (n = 28/79). The prevalence of different virulence genes is shown in Figure 1. With regard to both enterococcal species, the analysis showed that all selected virulence genes were positive among E. faecium isolates. The exception is hyl where it was detected with 82.7% prevalence rate. Among E. faecalis isolates, pilB, fms8, efaAfm, and sgrA genes were detected in all isolates, followed by scm (88%), ecbA (70%), and pilA (58%). The least prevalence was hyl which was detected in only 4 isolates (8%). Table 2 shows the distribution of different virulent determinants among E. faecium and E. faecalis clinical isolates. We believe that this is the first report on the prevalence of selected virulence genes among E. faecalis which could be demonstrated by the possibility of horizontal gene transfer among E. faecium and E. faecalis.
Figure 1.
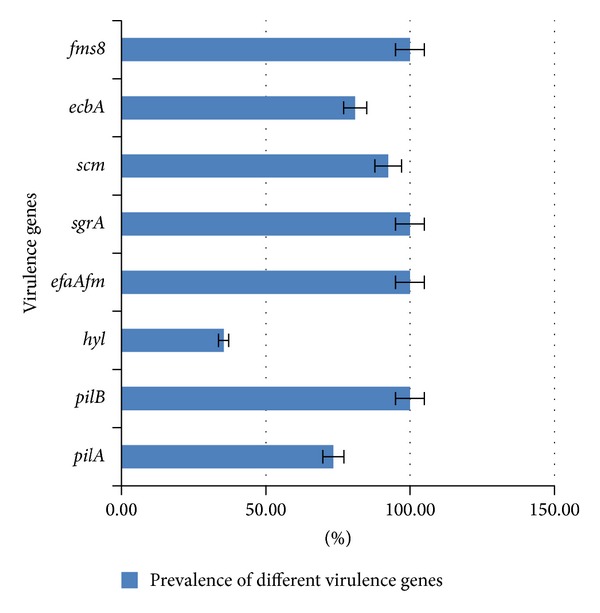
Prevalence of different virulence genes in clinical E. faecium and E. faecalis isolates. The pilB, fms8, efaAfm, and sgrA were identified in all clinical isolates.
Table 2.
Distribution of different virulence factors in E. faecium and E. faecalis.
| Virulence genes | E. faecalis | E. faecium | Total |
|---|---|---|---|
| (n = 50) | (n = 29) | (N = 79) | |
| pilB | 50 (100%) | 29 (100%) | 79 (100%) |
| fms8 | 50 (100%) | 29 (100%) | 79 (100%) |
| efaAfm | 50 (100%) | 29 (100%) | 79 (100%) |
| sgrA | 50 (100%) | 29 (100%) | 79 (100%) |
| scm | 44 (88%) | 29 (100%) | 73 (92.4%) |
| ecbA | 35 (70%) | 29 (100%) | 64 (81%) |
| pilA | 29 (58%) | 29 (100%) | 58 (73.4%) |
| hyl | 4 (8%) | 24 (82.7%) | 28 (35.4%) |
Pili which are also known as fimbriae have been detected in Gram-positive bacteria [13–15]. This surface organelle is responsible for endocarditis and biofilm formation in Gram-positive bacteria [16], and mediates attachment to human epithelium and skin [17] and confers resistance against macrophages [18].
So far, limited data has been documented on the prevalence of pili in E. faecium. In our study, high prevalence rate of pilB in clinical E. faecium and E. faecalis isolates (100% for each) has been observed. In comparison with pilB, 21 (26.6%) clinical E. faecalis isolates were negative for the presence of pilA. Figures 2 and 3 show the presence of pilA and pilB genes in representative isolates. Hendrickx et al. [19] have demonstrated the presence of pilA and pilB in clinical isolates of E. faecium. Their analysis showed that pilA could not be expressed in broth condition and the best temperature for their expression was 37°C. This could probably be a reason as to why we could not detect pilA in some of our clinical E. faecalis isolates. Another possible reason could be explained by the lack of horizontal gene transfer among E. faecalis isolates.
Figure 2.
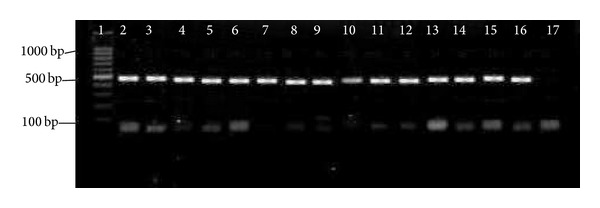
PCR results of pilA. 1 = marker = 100 bp; 2–16 = pilA = 495 bp; 17 = negative control.
Figure 3.
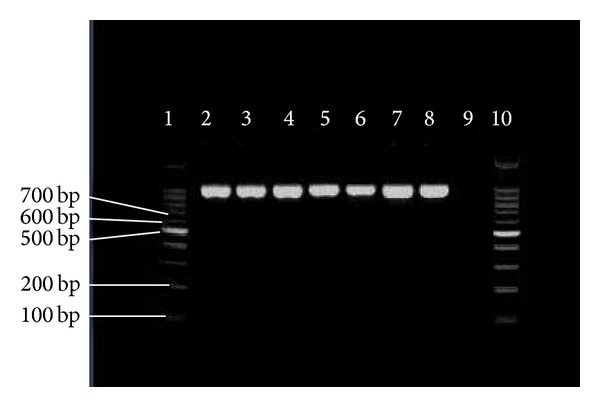
PCR results of pilB. 1, 10 = marker = 100 bp; 2–8 = pilB = 959 bp; 9 = negative control.
Nowadays, it is obvious that in pathogenic bacteria several proteins have evolved to adhere to and invade into the host cells and subsequently escape and resist host defense [20]. MSCRAMMs which are known as microbial surface components recognizing adhesive matrix molecules have an important role for cell adhesion and involve in pathogenicity of bacteria [21]. In silico analysis has revealed a family of genes that encode MSCRAMM-like proteins in Enterococcus such as ebp (endocarditis and biofilm-associated pilus of E. faecalis) operon, ace (adhesion of collagen from E. faecalis), and acm (adhesion of collagen from E. faecium) [22–24].
The role of collagen-binding MSCRAMM acm (Fms8) is to bind to the collagen types I and IV. These types of collagen are also an important antigen involved in human during endocarditis. In the current research, high prevalence rate of fms8 (100% for each) was detected among E. faecium and E. faecalis clinical isolates and could play an important role in pathogenicity of both enterococcal species. Figure 4 shows the presence of fms8 genes in representative isolates.
Figure 4.
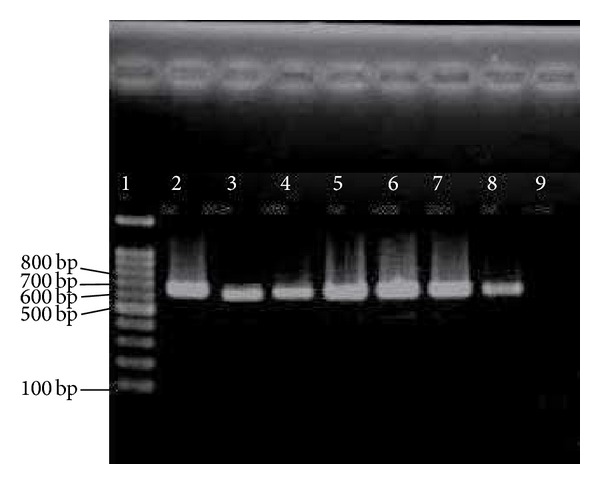
PCR results of fms8. 1 = marker = 100 bp; 2–8 = fms8 = 765 bp; 9 = negative control.
The exact role of efaAfm is unknown although it is believed to be involved in cell wall adherence. The efaAfm gene was only found in E. faecium isolates [25]. A study by Barbosa et al. [26] has demonstrated 27% of isolates harbored the efaAfm gene while the current study showed all isolates were positive for efaAfm. The presence of efaAfm gene among representative isolates is shown in Figure 5. Our finding corroborates with Martin et al. [27] where they have demonstrated all E. faecium isolates carrying efaAfm virulence gene.
Figure 5.
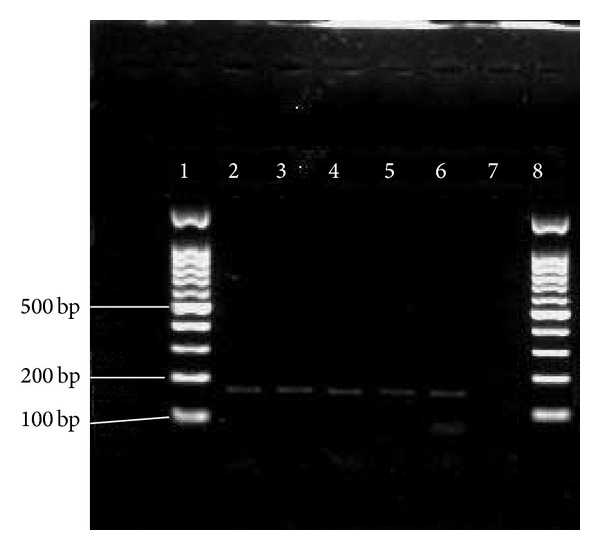
PCR results of efaAfm. 1, 8 = marker = 100 bp; 2–6 = efaAfm = 199 bp; 7 = negative control.
The pathogenic E. faecium is enriched with two orf2351 and orf2430 genes which encode the sgrA and ecbA LPXTG-like cell wall anchored proteins, respectively. sgrA is a surface adhesion that can bind to the extracellular matrix molecules nidogen 1 and nidogen 2 and is also involved in biofilm formation. Meanwhile, ecbA binds to the collagen type V and fibrinogen. Both ecbA and sgrA were reported to be prevalent in clinical strains of E. faecium [28]. However, our study demonstrated that sgrA was more prevalent than ecbA (100% versus 81%). The representative isolates for the presence of sgrA and ecbA genes are shown in Figures 6 and 7.
Figure 6.
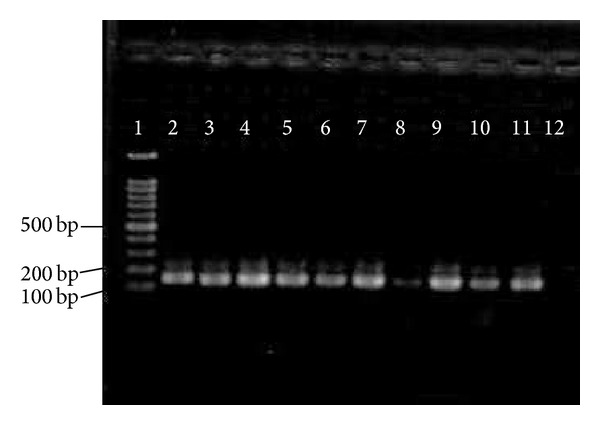
PCR results of sgrA. 1 = marker = 100 bp; 2–11 = sgrA = 150 bp; 12 = negative control.
Figure 7.
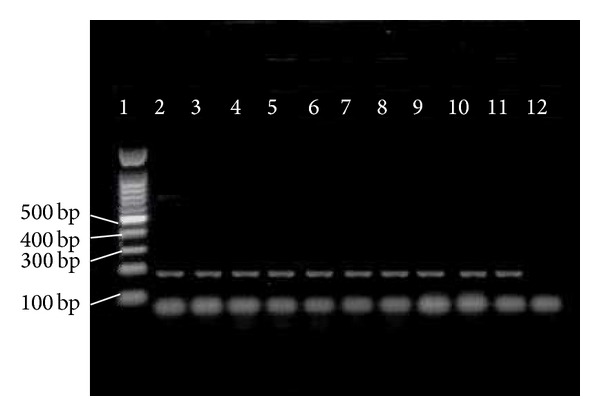
PCR results of ecbA. 1 = marker = 100 bp; 2–11 = ecbA = 182 bp; 12 = negative control.
In addition, hyl virulence gene encodes for a putative glycosyl hydrolase that is considered as a plasmid harboring gene which has colonized the gastrointestinal tracts of mice subsequently caused an increase of pathogenicity of E. faecium in experimental peritonitis [29]. In a study by Panesso et al. [30] that subjected 32 hospitals in Colombia, Ecuador, Peru, and Venezuela, the results revealed that 23% of E. faecium strains carried the hyl virulence gene. Our results are in accordance with the previous study by Panesso et al. [30]. The hyl gene among representative isolates is shown in Figure 8. In a research by Worth et al. [31] among Australian haematology patients, only 2.1% of isolates showed positive reaction to hyl. A study by Vankerckhoven et al. [32] revealed 71% of VRE and 29% of vancomycin sensitive Enterococcus (VSE) harbored hyl virulence gene. Since hyl is not prevalent in all clinical isolates in our study, we believe that this gene could has little role in pathogenicity of Enterococcus in comparison with other prevalent virulence genes.
Figure 8.
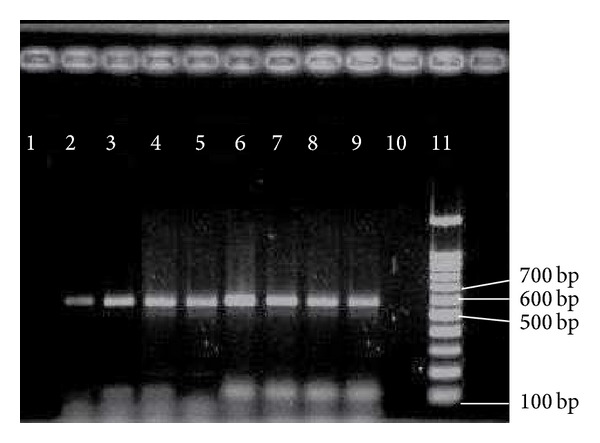
PCR results of hyl. 11 = marker = 100 bp; 2–9 = hyl = 605 bp; 1, 10 = negative control.
Scm (second collagen adhesion of E. faecium) binds to collagen type V and fibrinogen and it is commonly distributed among clinical and nonclinical isolates of E. faecium. Scm was first described in E. faecium in 2008 [33] but limited information on the prevalence of scm in E. faecium has been reported. Nonetheless, we documented a high prevalence of scm in clinical isolates of E. faecium (92.4%). Isolates representing for the presence of scm gene are shown in Figure 9.
Figure 9.
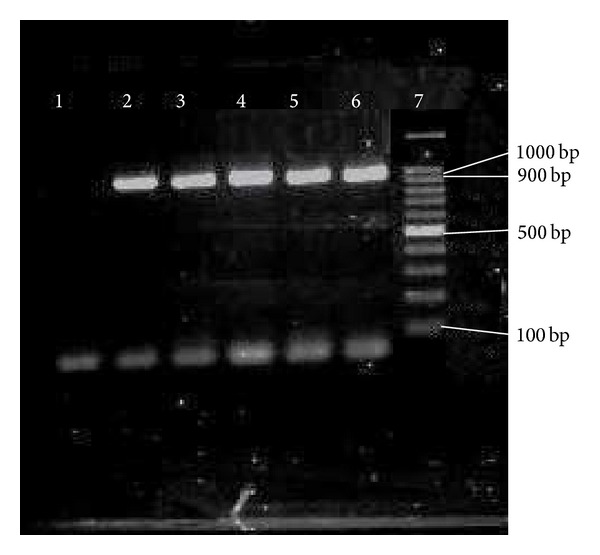
PCR results of scm. 7 = marker = 100 bp; 1–5 = scm = 1015 bp; 1 = negative control.
4. Conclusion
The wide distribution of several virulence genes that is pilB, fms8, efaAfm, and sgrA in E. faecalis and E. faecium clinical isolates could give a clue that these virulence genes might play an important role in the pathogenicity of both enterococcal species. This could also be explained that the horizontal virulence gene transfer is common among the clinical isolates.
Acknowledgment
This study was supported by Universiti Putra Malaysia, Research University Grant Scheme (RUGS), with Project no. 04-20-12-1766RU.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Shankar V, Baghdayan AS, Huycke MM, Lindahl G, Gilmore MS. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infection and Immunity. 1999;67(1):193–200. doi: 10.1128/iai.67.1.193-200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sava IG, Heikens E, Huebner J. Pathogenesis and immunity in enterococcal infections. Clinical Microbiology and Infection. 2010;16(6):533–540. doi: 10.1111/j.1469-0691.2010.03213.x. [DOI] [PubMed] [Google Scholar]
- 3.Vergis EN, Shankar N, Chow JW, et al. Association between the presence of enterococcal virulence factors gelatinase, hemolysin, and enterococcal surface protein and mortality among patients with bacteremia due to Enterococcus faecalis . Clinical Infectious Diseases. 2002;35(5):570–575. doi: 10.1086/341977. [DOI] [PubMed] [Google Scholar]
- 4.Top J, Willems R, Bonten M. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunology and Medical Microbiology. 2008;52(3):297–308. doi: 10.1111/j.1574-695X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 5.Weng PL, Ramli R, Shamsudin MN, Cheah Y-K, Hamat RA. High genetic diversity of Enterococcus faecium and Enterococcus faecalis clinical isolates by pulsed-field gel electrophoresis and multilocus sequence typing from a hospital in Malaysia. BioMed Research International. 2013;2013:6 pages. doi: 10.1155/2013/938937.938937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson AP. The pathogenicity of enterococci. Journal of Antimicrobial Chemotherapy. 1994;33(6):1083–1089. doi: 10.1093/jac/33.6.1083. [DOI] [PubMed] [Google Scholar]
- 7.Ike Y, Clewell DB, Segarra RA, Gilmore MS. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertion mutagenesis and cloning. Journal of Bacteriology. 1990;172(1):155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsen T, Nes IF, Holo H. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Applied and Environmental Microbiology. 2003;69(5):2975–2984. doi: 10.1128/AEM.69.5.2975-2984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su YA, Sulavik MC, He P, et al. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis subsp. liquefaciens . Infection and Immunity. 1991;59(1):415–420. doi: 10.1128/iai.59.1.415-420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An FY, Sulavik MC, Clewell DB. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. Journal of Bacteriology. 1999;181(19):5915–5921. doi: 10.1128/jb.181.19.5915-5921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe AM, Lambert PA, Smith AW. Cloning of an Enterococcus faecalis endocarditis antigen: homology with adhesins from some oral streptococci. Infection and Immunity. 1995;63(2):703–706. doi: 10.1128/iai.63.2.703-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton TJ, Gasson MJ. Molecular Screening of Enterococcus Virulence Determinants and Potential for Genetic Exchange between Food and Medical Isolates. Applied and Environmental Microbiology. 2001;67(4):1628–1635. doi: 10.1128/AEM.67.4.1628-1635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauer P, Rinaudo CD, Soriani M, et al. Microbiology: Genome analysis reveals pili in group B streptococcus. Science. 2005;309(5731):p. 105. doi: 10.1126/science.1111563. [DOI] [PubMed] [Google Scholar]
- 14.Yeung MK, Ragsdale PA. Synthesis and function of Actinomyces naeslundii T14V type 1 fimbriae require the expression of additional fimbria-associated genes. Infection and Immunity. 1997;65(7):2629–2639. doi: 10.1128/iai.65.7.2629-2639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung MK, Donkersloot JA, Cisar JO, Ragsdale PA. Identification of a gene involved in assembly of Actinomyces naeslundii T14V type 2 fimbriae. Infection and Immunity. 1998;66(4):1482–1491. doi: 10.1128/iai.66.4.1482-1491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nallapareddy SR, Murray BE. Ligand-signaled upregulation of Enterococcus faecalis ace transcription, a mechanism for modulating host-E. faecalis interaction. Infection and Immunity. 2006;74(9):4982–4989. doi: 10.1128/IAI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbot EL, Smith WD, Siou GPS, et al. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cellular Microbiology. 2007;9(7):1822–1833. doi: 10.1111/j.1462-5822.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 18.Maisey HC, Quach D, Hensler ME, et al. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. The FASEB Journal. 2008;22(6):1715–1724. doi: 10.1096/fj.07-093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrickx APA, Bonten MJM, van Luit-Asbroek M, Schapendonk CME, Kragten AHM, Willems RJL. Expression of two distinct types of pili by a hospital-acquired Enterococcus faecium isolate. Microbiology. 2008;154(10):3212–3223. doi: 10.1099/mic.0.2008/020891-0. [DOI] [PubMed] [Google Scholar]
- 20.Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124(4):715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-medicated adherence of microorganisms to host tissues. Annual Review of Microbiology. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 22.Bowden MG, Chen W, Singvall J, et al. Identification and preliminary characterization of cell-wall-anchored proteins of Staphylococcus epidermidis. Microbiology. 2005;151(5):1453–1464. doi: 10.1099/mic.0.27534-0. [DOI] [PubMed] [Google Scholar]
- 23.Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infection and Immunity. 2000;68(9):5218–5224. doi: 10.1128/iai.68.9.5218-5224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Liang X, Chen Y, Koehler TM, Höök M. Identification and biochemical characterization of two novel collagen binding MSCRAMMs of Bacillus anthracis. Journal of Biological Chemistry. 2004;279(50):51760–51768. doi: 10.1074/jbc.M406417200. [DOI] [PubMed] [Google Scholar]
- 25.Martín M, Gutiérrez J, Criado R, Herranz C, Cintas LM, Hernández PE. Genes encoding bacteriocins and their expression and potential virulence factors of enterococci isolated from wood pigeons (Columba palumbus) Journal of Food Protection. 2006;69(3):520–531. doi: 10.4315/0362-028x-69.3.520. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa J, Ferreira V, Teixeira P. Antibiotic susceptibility of enterococci isolated from traditional fermented meat products. Food Microbiology. 2009;26(5):527–532. doi: 10.1016/j.fm.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Martin B, Garriga M, Hugas M, Aymerich T. Genetic diversity and safety aspects of enterococci from slightly fermented sausages. Journal of Applied Microbiology. 2005;98(5):1177–1190. doi: 10.1111/j.1365-2672.2005.02555.x. [DOI] [PubMed] [Google Scholar]
- 28.Hendrickx APA, Van Luit-Asbroek M, Schapendonk CME, et al. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium . Infection and Immunity. 2009;77(11):5097–5106. doi: 10.1128/IAI.00275-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias CA, Panesso D, Singh KV, Rice LB, Murray BE. Cotransfer of antibiotic resistance genes and a hylEfm-containing virulence plasmid in Enterococcus faecium . Antimicrobial Agents and Chemotherapy. 2009;53(10):4240–4246. doi: 10.1128/AAC.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panesso D, Reyes J, Rincón S, et al. Molecular epidemiology of vancomycin-resistant Enterococcus faecium: a prospective, multicenter study in South American hospitals. Journal of Clinical Microbiology. 2010;48(5):1562–1569. doi: 10.1128/JCM.02526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Worth LJ, Slavin MA, Vankerckhoven V, Goossens H, Grabsch EA, Thursky KA. Virulence determinants in vancomycin-resistant Enterococcus faecium vanB: clonal distribution, prevalence and significance of esp and hyl in Australian patients with haematological disorders. Journal of Hospital Infection. 2008;68(2):137–144. doi: 10.1016/j.jhin.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Vankerckhoven V, Van Autgaerden T, Vael C, et al. Development of a multiplex PCR for the detection of asaI, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium . Journal of Clinical Microbiology. 2004;42(10):4473–4479. doi: 10.1128/JCM.42.10.4473-4479.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sillanpää J, Nallapareddy SR, Prakash VP, et al. Identification and phenotypic characterization of a second collagen adhesin, Scm, and genome-based identification and analysis of 13 other predicted MSCRAMMs, including four distinct pilus loci, in Enterococcus faecium . Microbiology. 2008;154(10):3199–3211. doi: 10.1099/mic.0.2008/017319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


