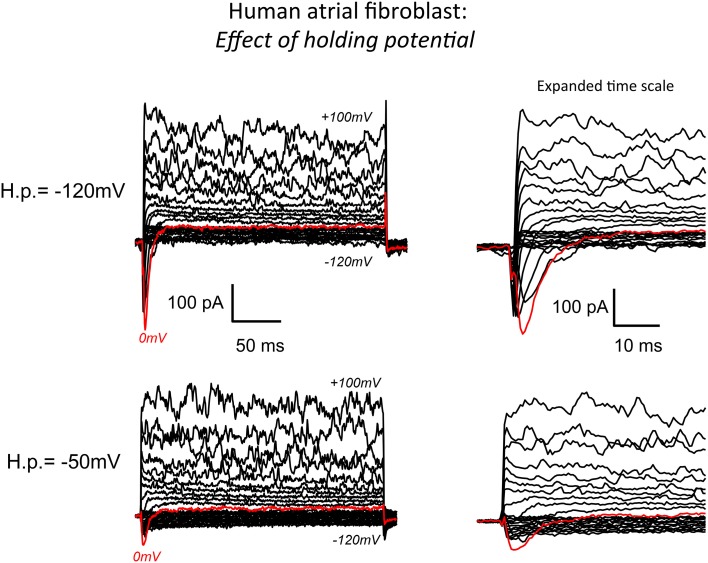
Figure 1.
Whole cell patch clamp records of the predominant transmembrane ionic currents recorded from an isolated human atrial myofibroblast using our standard superfusion solution at 22–23°C. The pipette solution in this experiment was “K+-rich.” (See Methods for details of solutions and voltage-clamp protocols). The superimposed current records in the bottom left panel are typical of the data recorded when the voltage clamp “holding potential” (−50 mV) was set near the presumed resting membrane potential of the myofibroblast (approximately −40 mV). As shown, membrane depolarizations in the voltage range from about +20 to 100 mV resulted in a large “noisy” outward current that is likely generated by Ca2+-activated K+ channels. In this and most other recordings, hyperpolarization from HP −50 mV produced only very small current changes (see Chilton et al., 2007). Note, however, that depolarizing steps did elicit (red trace) a small but distinct transient inward current. The same records at an expanded time scale are shown on the right. The data in the top panel were obtained in response to the same levels of step depolarizations applied from a holding potential of −120 mV. As expected from the fundamental biophysical properties of a voltage-dependent Na+ (or Ca2+) current this hyperpolarized HP “unmasked” a much more prominent transient inward current (see Results).