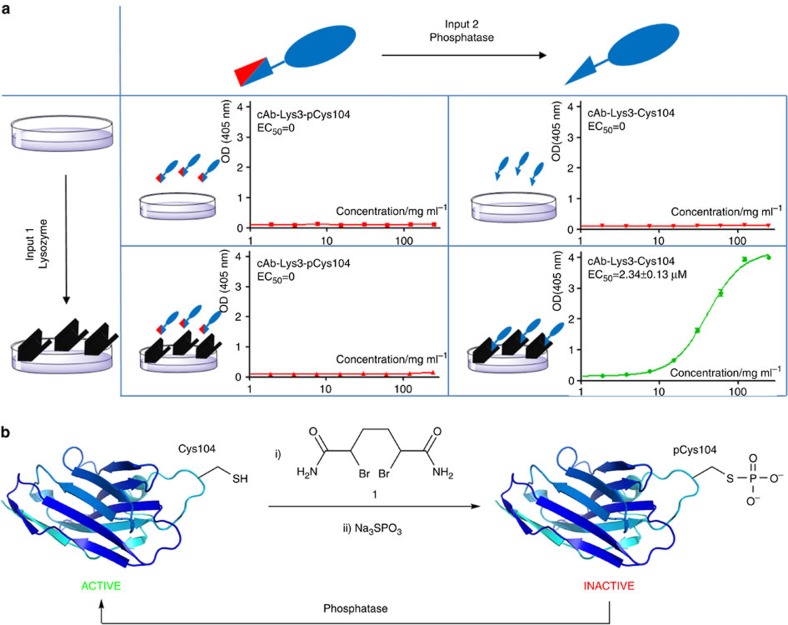
Figure 1. Design and construction of a ‘gated’ antibody.
(a) The gated two-input activation of an AND antibody based upon cAbLys3. Input 1 is presentation of the corresponding selective antigen (black shape, here lysozyme) that engages as a ligand for the Complementarity Determining Region (CDR) in its unblocked state. Input 2 is the presence of the enzyme that unblocks that CDR by removing a blocking group (red shape, here removed by phosphatase). Enzyme-linked immunosorbent assay data for binding shows functional ‘output’ only upon the presence of both inputs (green). All other input states fail to generate activity (red). (b) The gated AND antibody that responds to this input can be constructed in a highly stable, single-chain form through site-selective, chemical phosphorylation within the CDR to block its binding function. Active cAbLys3 containing an engineered Cys residue at residue 104 (A104C) was reacted first with the HDADB reagent (1) to create unnatural amino acid Dha (step i) and then with sodium thiophosphate (step ii) to introduce phosphoryl as a blocking group thereby rendering the Ab inactive. The phosphoryl block group is removed by the action of phosphatase-catalyzed dephosphorylation to unblock the CDR and regenerate active antibody binding as an output.