Figure 1. Adverse Events.
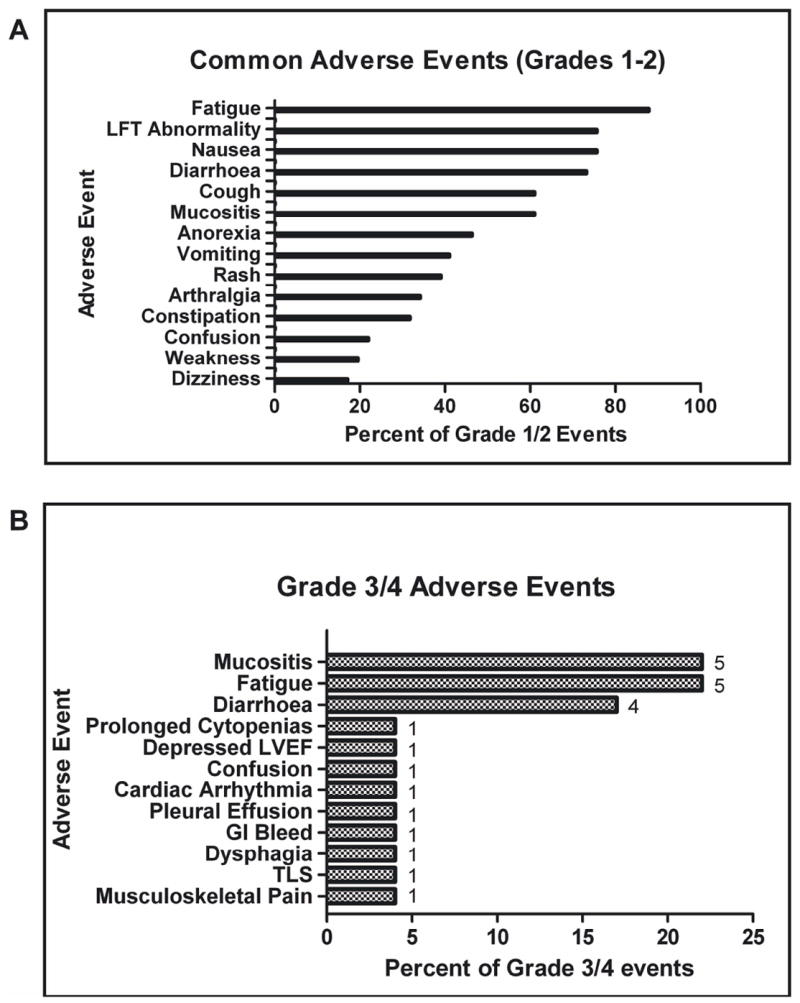
A. Most common grade 1 or 2 non-haematological adverse events seen in patients on the trial. B. Most common grade 3 or 4 adverse events seen in patients on the trial. The bar graphs express a percentage. The numbers next to each bar represent actual numbers of events. LVEF, left ventricular ejection fraction; GI, gastrointestinal; TLS, tumour lysis syndrome.
