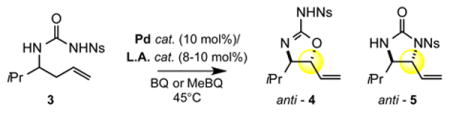
Table 1. Reaction Development.
| |||||||
|---|---|---|---|---|---|---|---|
| entrya,b | R |
Pd catalyst (10 mol%) |
L.A. co-catalyst (8 - 10 mol%) |
Yield of 4 (%)c,d |
Yield of 5 (%)c,d |
4:5 | |
| 1e | Ns | 1 | none | 19% | 28% | 1:1.6 |
|
| 2f | Ns | 1 | DIPEA | 0% | 38% | 1:>20 | |
| 3e | Ns | 1 | B(C6F6)3 | 50% | 7% | 4.9:1 | |
| 4g | Ns | 1 | AgOTf | 76% | 9% | 7:1 | |
| 5h | Ns | 1 | Cr(salen)Cl | 8% | 8% | 1:1 | |
| 6g | Ts | 1 | AgOTf | 56% | trace | >20:1 | |
| 7g | Ns | 1 | TfOH | 64% | 28% | 2.3:1 | |
| 8i | Ns | none | TfOH | 0% | 0% | - | |
| 9g,i | Ns | none | AgOTf | 0% | 0% | - | |
| 10e | Ns | 2 | AgOTf | 0% | 46% | 1:>20 | Pd(OAc)2 cat. 2 |
| 11 j | Ns | 2 | AgOTf | 0% | 59% | 1:>20 | |
| 12j | Ns | 2 | B(C6F6)3 | 0% | 62% | 1:>20 | |
| 13j | Ns | 2 | none | 0% | 32% | 1:>20 | |
| 14i,j | Ns | none | B(C6F6)3 | 0% | 0% | - | |
All reactions were run using THF (1.0M) as solvent, MeBQ (methyl-p-benzoquinone, 1.5 equiv.) as terminal oxidant and 8 mol% L.A. co-catalyst for 6 hours unless otherwise noted.
Reactions with cat. 1 were run with an additional 5 mol% BisSO ligand [1,2-bis(phenylsulfinyl)ethane].
Average of 2 runs.
Observed diastereoselectivities in crude reaction mixtures: >20:1 dr for 4 for all entries; dr for 5: entry 1, 1.3:1 dr anti:syn; entry 2, 4.4:1 dr; entry 4, 1:1 dr; entries 3, 5, 10, 11, 12, 13 >20:1 dr; dr determined by 1H NMR analysis of the crude reaction mixture.
Reactions run to complete conversion: entry 1, 24h; entry 3, 2h; entry 10, 72h.
Reaction stopped at 18h (37% rsm); no improved conversion was observed at 48h or 72h.
DCM (dichloromethane, 1.0M); For entry 4, 1 equiv. of DMA was added.
Reaction stopped before complete conversion at 2h. Increased reaction times resulted in product decomposition.
>99% rsm, 0% E-internal olefin.
Reaction run using THF (1.66M) as solvent, BQ (p-benzoquinone, 1.05 equiv.) as terminal oxidant and 10 mol% L.A. co-catalyst for 72 hours.
