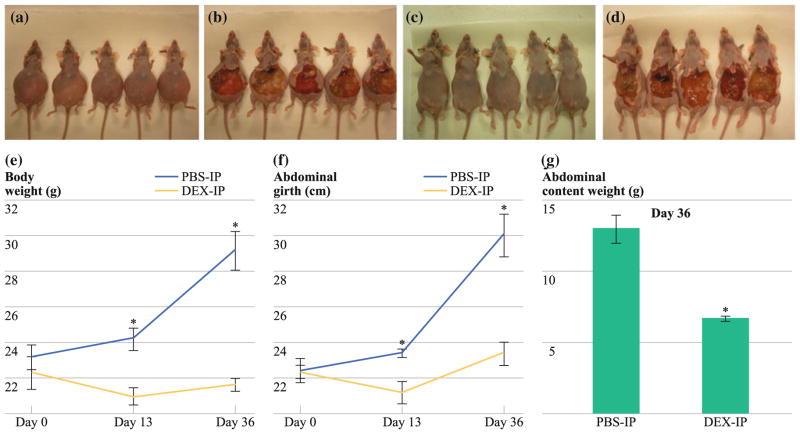
FIG. 3.
Intraperitoneal PMP xenograft model showing decreased abdominal distention and mucinous tumor at days 13 and 36 after Dex treatment. a PBS-IP, before laparotomy; b PBS-IP, after laparotomy; c Dex-IP, before laparotomy; and d Dex-IP, after laparotomy; all mice were euthanized on day 36. Chronic treatment with Dex (2 mg/kg/day, IP) led to a serial reduction in (e) gross body weight (day 36: PBS-IP: 29.2 ± 1.09 g vs. Dex-IP: 21.7 ± 0.34 g; P = 0.002), f abdominal girth (day 36: PBS-IP: 30.1 ± 1.2 cm vs. Dex-IP: 23.4 ± 0.63 cm; P = 0.003), and g weight of en bloc abdominal contents (day 36: PBS-IP: 13.02 ± 0.96 g vs. Dex-IP: 6.72 ± 0.17 g; P = 0.002). Error bars denote SEM (n = 5); means were compared using paired Student’s t test; *P < 0.05