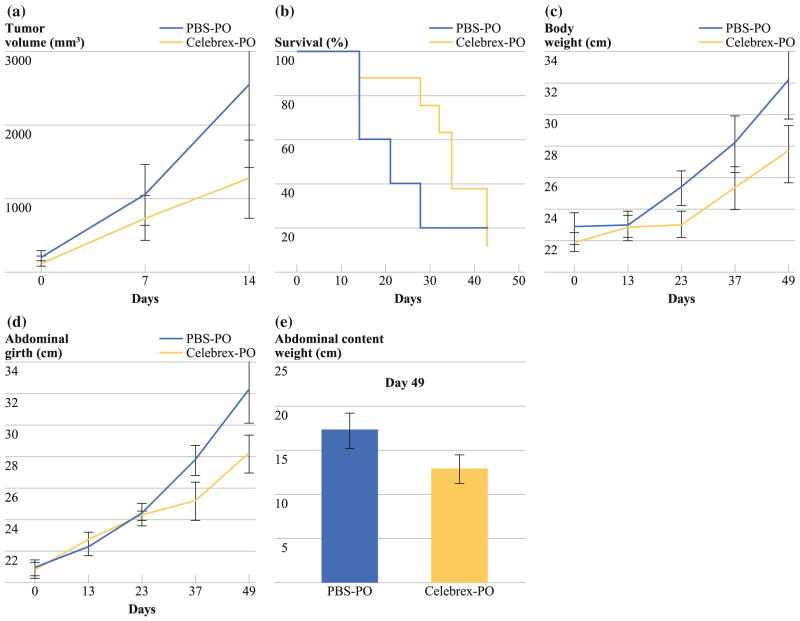
FIG. 6.
Treatment of subcutaneous LS174T murine xenograft model with Celebrex. a Statistically nonsignificant trend toward reduced subcutaneous tumor volume over time in Celebrex-treated mice compared with PBS-treated mice (day 14: PBS-PO: 2,550 ± 1,129 mm3 vs. Celebrex-PO: 1,264 ± 532 mm3; P > 0.05). b The survival curve reports the number of mice still alive on different days. Data refer to five PBS-treated and eight Celebrex-treated mice from day 1 of therapy until death. There was a trend toward longer median survival in Celebrex-treated mice (PBS-PO: 18 days vs. Celebrex-PO: 34 days; log-rank test, P > 0.05). Intraperitoneal PMP xenograft model treated with Celebrex (20 mg/kg/day, PO) demonstrated a statistically nonsignificant serial reduction in (c) gross body weight (PBS-PO: 30.5 ± 2.5 g vs. Celebrex-PO: 26.6 ± 1.7 g; P >0.05), d abdominal girth (PBS-PO: 32.2 ± 2.1 cm vs. Celebrex-PO: 28.1 ± 1.2 cm; P > 0.05), and (e) weight of en bloc abdominal contents (organ block + mucinous tumor; PBS-PO: 17.4 ± 1.9 g vs. Celebrex-PO: 13.1 ± 1.6 g; P > 0.05) at the time they were euthanized. Error bars denote SEM (n = 5); means were compared using paired Student’s t test