Table 1. Model parameter estimates obtained for different drug treatments of chronic HCV.
| Treatment |
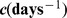
|
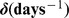
|
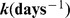
|
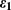
|
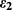
|
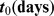
|

|
Trial Dur. | Source |
| Telaprevir | 13.4 | 0.58 | 2.86 | 0.974 | 0.999 | 0.10 | n/a | 2.5 days | [6] |
| Mericitabine, qd 750 mg | 6* | 0.023 | 1.06 (flat) | 0.38 | 0.86 | 0.37 | n/a | 2 weeks | [16] |
| 0.23 (non-flat) | |||||||||
| Mericitabine, qd 1500 mg | 6* | 0.023 | 1.06 (flat) | 0.38 | 0.94 | 0.37 | n/a | 2 weeks | [16] |
| 0.23 (non-flat) | |||||||||
| Mericitabine, bid 750 mg | 6* | 0.023 | 2.03 (flat) | 0.38 | 0.98 | 0.37 | n/a | 2 weeks | [16] |
| 0.43 (non-flat) | |||||||||
| Mericitabine, bid 1500 mg | 6* | 0.023 | 2.03 (flat) | 0.38 | 0.998 | 0.37 | n/a | 2 weeks | [16] |
| 0.43 (non-flat) | |||||||||
| Silibinin | 6* | 0.62 | 2.12 | n/a | 0.861 | n/a | 6** | 7 days | [19] |
| Danoprevir, bid 100 mg | 7.25 | 0.184 | 29.1 | n/a | 0.973 | n/a | n/a | 13 days | *** |
| Danoprevir, bid 200 mg | 7.25 | 0.184 | 29.1 | n/a | 0.985 | n/a | n/a | 13 days | *** |
| Danoprevir, bid 300 mg | 7.25 | 0.184 | 29.1 | n/a | 0.99 | n/a | n/a | 13 days | *** |
| Sofosbuvir | 5.76 | 0.53 | 8.12 | n/a | 0.998 | n/a | n/a | 7 days | *** |
Model parameter  gives the viral clearance rate,
gives the viral clearance rate,  the infected hepatocyte death rate,
the infected hepatocyte death rate, 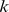 gives the exponential scale at which the drug reaches its maximum value
gives the exponential scale at which the drug reaches its maximum value 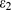 from its minimum value
from its minimum value 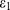 ,
, 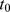 gives the delay in the drug activity, and
gives the delay in the drug activity, and 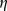 gives the efficacy of treatment in blocking new cell infection.
gives the efficacy of treatment in blocking new cell infection.
*Clearance rate  fixed at
fixed at 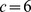 days−1 from [3], not estimated.
days−1 from [3], not estimated.
**Efficacy of treatment in blocking new cell infection 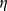 fixed at
fixed at 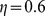 from [16], not estimated.
from [16], not estimated.
***Unpublished.
Notes: (i) In fitting viral load data, authors investigating telaprevir and mericitabine used the more general VE model (12), while those investigating silibinin, danoprevir, and sofosbuvir used the simple VE model (3) with 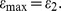 Efficacy of treatment in blocking new cell infection
Efficacy of treatment in blocking new cell infection 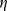 was only used in the silibinin model (effectively 0 in other models). (ii) qd = daily dosing, bid = bi-daily dosing. (iii) Parameter estimates derive from HCV treatment studies on patients who were treatment naïve, except in the case of mericitabine, where all patients were interferon non-responders.
was only used in the silibinin model (effectively 0 in other models). (ii) qd = daily dosing, bid = bi-daily dosing. (iii) Parameter estimates derive from HCV treatment studies on patients who were treatment naïve, except in the case of mericitabine, where all patients were interferon non-responders.
