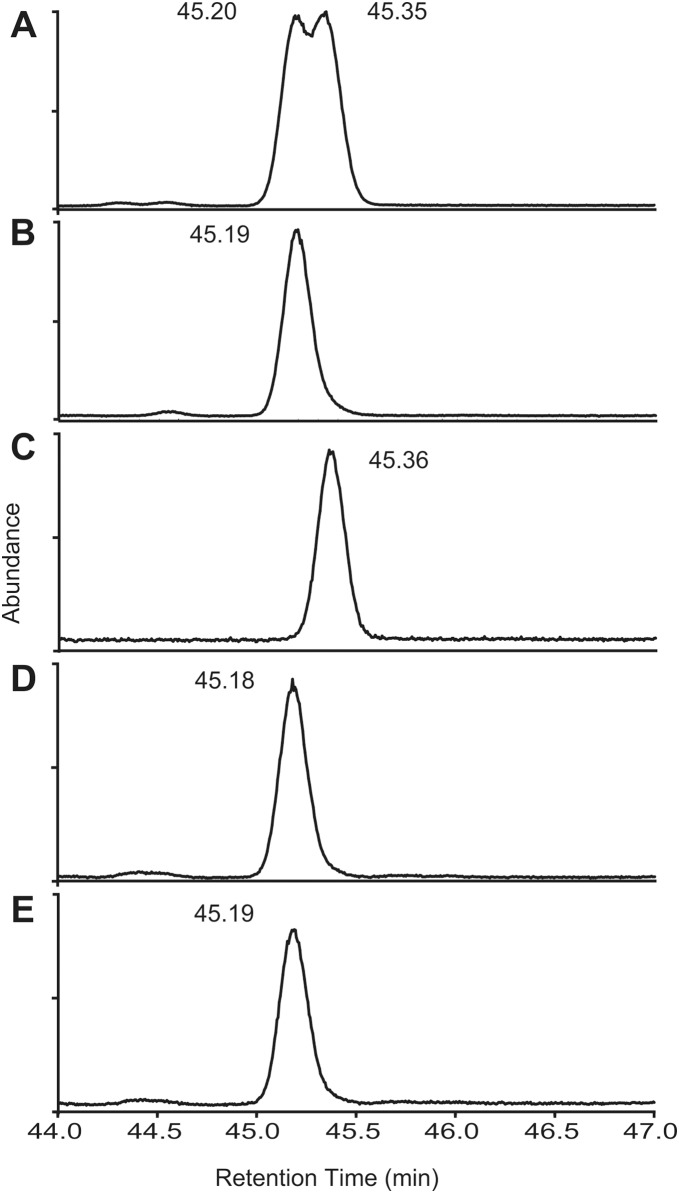
Figure 1. GC/MS analysis on a chiral column.
Confirmation of Thrips palmi aggregation pheromone as the (R) enantiomer by GC/MS analysis on a CycloSil-B analytical column: (A) section of the TIC chromatogram from 44 to 47 min showing the two partly resolved peaks obtained from racemic lavandulyl 3-methyl-3-butenoate; (B) the peak obtained on injection of the (R)-lavandulyl 3-methyl-3-butenoate enantiomer; (C) the peak obtained on injection of the (S)-lavandulyl 3-methyl-3-butenoate enantiomer; (D) the peak obtained on injection of the T. palmi natural compound; (E) the enhanced peak obtained on co-injection of the T. palmi natural compound and (R)-lavandulyl 3-methyl-3-butenoate.