Figure 3. Functional analysis of tooth agenesis-causing MSX1 mutations.
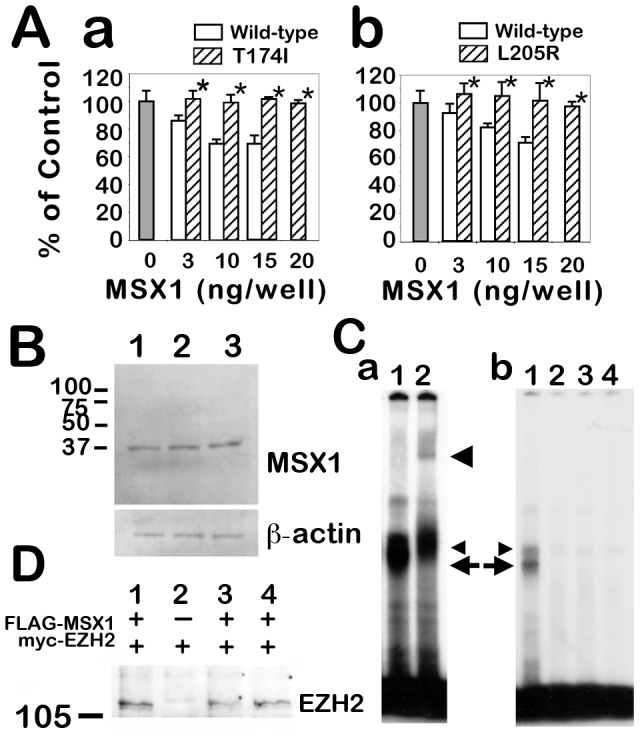
A: myoD-promoter repression activity of T174I and L205R variants in C2C12 cells. The C2C12 cells were cotransfected with an MSX1 expression vector, the firefly luciferase reporter gene driven by the myoD-promoter and sea pansy luciferase reporter gene driven by the thymidine kinase promoter. After differentiation treatments, firefly luciferase activity was measured and normalized with respect to the sea pansy luciferase activity. Open bars indicate human wild-type MSX1 transfectant data (3–15 ng/well); hatched bars denote data from experiments utilizing transfectants of the T174I (a) and L205R (b) variants of MSX1 (3–20 ng/well), respectively. The relative luciferase activity is expressed as a percentage of the negative control (gray bars). Each transfection point was carried out in triplicate in a gelatin-coated 24-well plate. The experiments were performed three times and representative data are shown. Error bars indicate standard deviation. Asterisks indicate no significant differences against the control (P-value >0.1). B: Stability and expression level of wild-type and mutant proteins. Western blot analysis was performed using extracts prepared from HEK293 cells transfected with FLAG-wild-type-MSX1 (lane 1), FLAG-T174I-MSX1 (lane 2) or FLAG-L205R-MSX1 (lane 3). These whole-cell extracts were immunoblotted with an anti-FLAG antibody (upper panel) and anti-beta actin antibody as an internal standard (lower panel). C: Electrophoretic mobility shift assay of MSX1. a: A double-stranded oligonucleotide probe containing a consensus binding-site for MSX1 interacts with proteins in the wild-type MSX1 transfectant cell lysate (lane 1, arrow and small arrowhead). The DNA binding ability of MSX1 is diminished by the T174I and L205R substitutions. Wild-type-MSX1 protein and oligo DNA complex observed in lane 1 (arrow) was not detected in the MSX1 variant transfectant lysates (lane 2, T174I-MSX1; lane 3, L205R-MSX1), or HEK293 parental cells (lane 4, negative control). b: Supershift experiments using an anti-FLAG antibody. The binding specificity was confirmed by a super-shift induction with anti-FLAG antibody (lane 2). A protein-DNA-antibody complex formed (large arrowhead), and the band indicated by the arrow disappeared. This indicates that this molecular complex denoted by the arrow contained FLAG-tagged wild-type MSX1 protein, and that the protein indicated by the small arrowhead is an unknown product that bound to the probe. D: Molecular interaction between MSX1 and EZH2 histone methyltransferase. Myc-EZH2 protein was coimmunoprecipitated with FLAG-MSX1 (lane 1). EZH2 protein was not detected in the MSX1-null EZH2 transfectant HEK293 cell lysate (lane 2). The MSX1-EZH2 interaction was observed in the FLAG-T174I-MSX1 sample (lane 3), and in the FLAG-L205R-MSX1 sample (lane 4).
