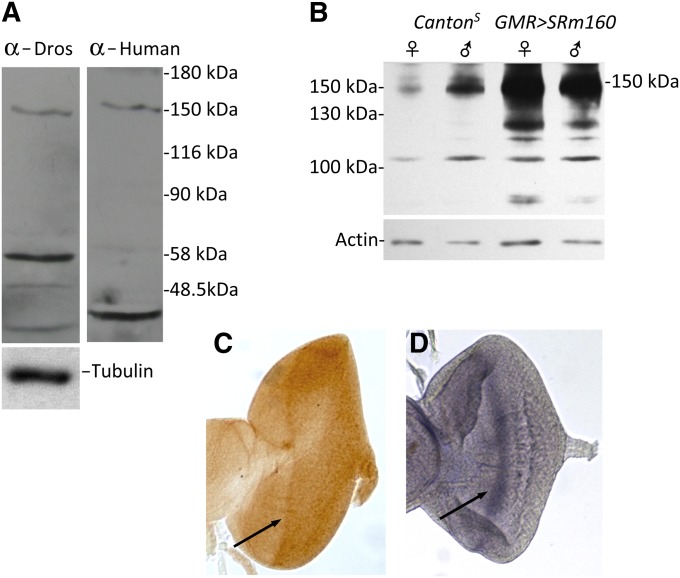
Figure 2.
Anti-human SRm160 recognizes Drosophila SRm160. (A) The same protein of 150 kDa is recognized by both anti-human anti-SRm160 antibody and anti-Drosophila SRm160. Protein samples are from adult flies. The transfer was stripped in between probing and no signal was detected following stripping, prior to redetection with the second antiserum. Lower-molecular-weight bands detected by the two sera may be SRm160 degradation products, because the protein is very labile and is undetectable if samples are boiled prior to loading (D. Gatfield, personal communication, confirmed by our results). Importantly, while a protein of identical molecular weight is observed at 150 kDa in both samples, the secondary bands are different, demonstrating no carryover of signal between probings of the blot with different sera. (B) GMR > SRm160 expression induces SRm160 protein overaccumulation (150 kDa) in head protein extracts compared with wild type (CS). The immunoblot was probed with anti-Drosophila SRm160. (C) An anti-SRm160 antibody-stained third-instar eye antennal disc shows higher-protein-level accumulation at the level of the morphogenetic furrow (arrow). (D) In situ hybridization of a third-instar SRm160 anti-sense probe hybridized to an eye antennal disc, revealing higher transcript accumulation in the morphogenetic furrow (arrow), confirming the specificity of the anti-human SRm160 for the Drosophila protein and also demonstrating increased concentration of the protein as well as the RNA in cells initially differentiating to the neuronal cell fate.