Table 1. Modulatory Effect of the Library of Small Molecule Analogues on TLR-Dependent Cytokine Productiona.
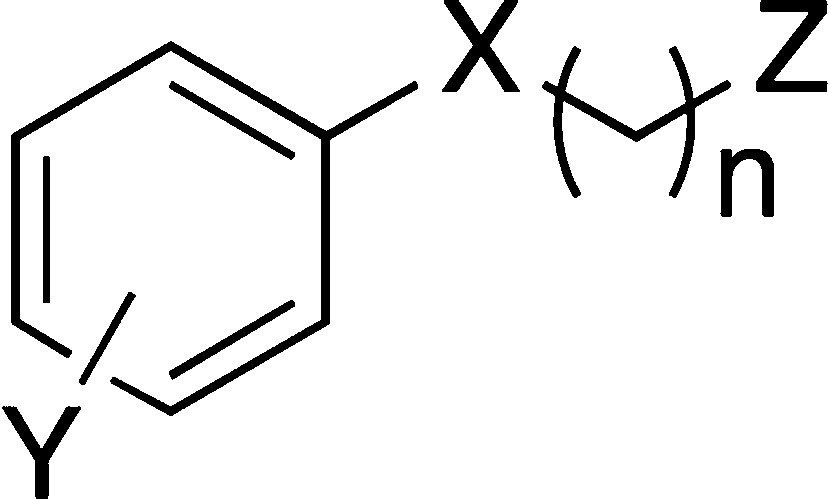
| (A) phosphonates and phosphates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMA | X | Y | n | Z | LPS IL-12 | LPS IL-6 | BLP IL-12 | BLP IL-6 | CPG IL-12 | CPG IL-6 |
| 5a | CH2PO3– | 4-Br | 2 | NMe3+ | ↓ | ↑ | ↓ | |||
| 5b | CH2PO3– | 4-Br | 2 | NMe2 | ↑ | ↑ | ||||
| 5c | CH2PO3– | 4-Me | 2 | NMe2 | ↑ | |||||
| 5d | CH2PO3– | 4-NO2 | 2 | NMe3+ | ↑ | ↑ | ||||
| 5e | CH2PO3– | 4-Me | 2 | NMe2 | ↑ | ↑ | ||||
| 6 | OPO3– | 4-BOCNH | 3 | NMe2 | ↑ | ↑ | ||||
| (B) sulfones | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMA | X | Y | n | Z | LPS IL-12 | LPS IL-6 | BLP IL-12 | BLP IL-6 | CpG IL-12 | CpG IL-6 |
| 11a | CH2SO2 | 4-Br | 2 | Me2N | ↓ | ↓ | ↓ | ↓ | ↓ | |
| 11b | CH2SO2 | 4-Me | 2 | Me2N | ↓ | ↓ | ||||
| 11c | CH2SO2 | 4-Me | 2 | pyrrol | ↓ | ↓ | ↓ | ↓ | ||
| 11d | CH2SO2 | 4-Br | 2 | pyrrol | ↓ | |||||
| 11e | CH2SO2 | 3-F | 2 | NMe2 | ↑ | ↑ | ||||
| 11f | CH2SO2 | 3-F | 2 | diam | ||||||
| 11g | CH2SO2 | 3-F | 2 | morph | ↑ | |||||
| 11h | CH2SO2 | 3-F | 2 | pyrrol | ↑ | ↓ | ||||
| 11i | CH2SO2 | 4-F | 2 | NMe2 | ↓ | ↓ | ||||
| 11j | CH2SO2 | 4-F | 2 | morph | ↓ | ↑ | ↑ | |||
| 11k | CH2SO2 | 4-F | 2 | pyrrol | ↑ | ↓ | ||||
| 11l | CH2SO2 | 4-F | 2 | diam | ↓ | |||||
| 11m | CH2SO2 | 3-F | 2 | S-2-Me-but | ||||||
| 11n | CH2SO2 | 3-F | 2 | R-2-Me-but | ↓ | ↓ | ||||
| 11o | CH2SO2 | 4-Br | 2 | S-2-Me-but | ↓ | |||||
| 11p | CH2SO2 | 4-Br | 2 | R-2-Me-but | ||||||
| 12a | CH2SO2 | 4-Br | 2 | Me3N+ | ||||||
| 12b | CH2SO2 | 4-Me | 2 | Me3N+ | ↓ | ↓ | ↓ | ↓ | ||
| 12c | CH2SO2 | 4-Me | 2 | pyrrol Me+ | ↓ | ↓ | ||||
| 12d | CH2SO2 | 4-Br | 2 | pyrrol Me+ | ↓ | ↓ | ↓ | |||
| 16a | CH2SO2 | 4-Br | 3 | pyrrol | ||||||
| 16b | CH2SO2 | 4-Br | 3 | NMe2 | ↓ | |||||
| 16c | CH2SO2 | 4-Br | 3 | morph | ↑ | ↓ | ||||
| 16d | CH2SO2 | 4-Me | 3 | pyrrol | ||||||
| 16e | CH2SO2 | 4-Me | 3 | NMe2 | ||||||
| 16f | CH2SO2 | 4-Me | 3 | morph | ||||||
| 17a | CH2SO2 | 4-Br | 3 | morph Me+ | ↑ | |||||
| 17b | CH2SO2 | 4-Br | 3 | Me3N+ | ↓ | ↓ | ||||
| 17c | CH2SO2 | 4-Br | 3 | pyrrol Me+ | ||||||
| 17d | CH2SO2 | 4-Me | 3 | pyrrol Me+ | ||||||
| 17e | CH2SO2 | 4-Me | 3 | Me3N+ | ||||||
| 18a | SO2 | H | 2 | diam | ↑ | |||||
| 18b | SO2 | H | 2 | thiomorph | ↓ | ↓ | ↑ | |||
| 18c | SO2 | H | 2 | morph | ↑ | ↓ | ||||
| 18d | SO2 | H | 2 | pyrrol | ↑ | ↓ | ||||
| 18e | SO2 | H | 2 | R-2-Me-but | ↑ | ↑ | ||||
| 18f | SO2 | H | 2 | S-2-Me-but | ||||||
| 18g | SO2 | H | 2 | diam (−Me) | ||||||
| 18h | SO2 | H | 2 | n-Pr | ↑ | ↑ | ↓ | ↓ | ||
| 18i | SO2 | H | 2 | i-Pr | ↓ | |||||
| 18j | SO2 | H | 2 | 3-OH-n-Pr | ↑ | ↑ | ↓ | |||
| 18k | SO2 | H | 2 | t-Bu | ↑ | ↓ | ||||
| 18l | SO2 | H | 2 | 4-Me-pip | ||||||
| (C) sulfonamides
and carboxamides | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMA | X | Y | n | Z | LPS IL-12 | LPS IL-6 | BLP IL-12 | BLP IL-6 | CpG IL-12 | CpG IL-6 |
| 19a | CH2SO2NH | H | 2 | NMe2 | ↑ | |||||
| 19b | CH2SO2NH | H | 2 | pyrrol | ||||||
| 19c | CH2SO2NH | H | 2 | morph | ||||||
| 19e | CH2SO2NH | 4-Br | 2 | NMe2 | ↑ | ↑ | ||||
| 19d | CH2SO2NH | 4-Br | 2 | pyrrol | ↑ | |||||
| 19f | CH2SO2NH | 4-Br | 2 | morph | ↑ | ↑ | ||||
| 19g | CH2SO2NH | 4-F | 2 | NMe2 | ↑ | ↑ | ||||
| 19h | CH2SO2NH | 4-F | 2 | pyrrol | ↑ | ↑ | ↑ | ↑ | ||
| 19i | CH2SO2NH | 4-F | 2 | morph | ↑ | ↑ | ||||
| 19j | CH2SO2NH | 4-Me | 2 | NMe2 | ↑ | |||||
| 19k | CH2SO2NH | 4-Me | 2 | pyrrol | ||||||
| 19l | CH2SO2NH | 4-Me | 2 | morph | ||||||
| 19m | CH2SO2NH | 4-NO2 | 2 | NMe2 | ↓ | |||||
| 19n | CH2SO2NH | 4-NO2 | 2 | pyrrol | ||||||
| 19o | CH2SO2NH | 4-NO2 | 2 | morph | ||||||
| 19p | CH2SO2NH | 4-Br | 3 | NMe2 | ||||||
| 19q | CH2SO2NH | 4-NO2 | 3 | NMe2 | ↓ | |||||
| 19r | CH2SO2NH | 4-Me | 3 | NMe2 | ||||||
| 19s | CH2SO2NH | 4-F | 3 | NMe2 | ||||||
| 19t | CH2SO2NH | H | 3 | NMe2 | ↑ | |||||
| 19u | CH2SO2NH | 2-naphthyl | 2 | morph | ↓ | ↓ | ↓ | ↓ | ||
| 19v | CH2SO2NH | 2-naphthyl | 2 | pyrrol | ↓ | |||||
| 19w | CH2SO2NH | 2-naphthyl | 2 | NMe2 | ||||||
| 19x | CH2SO2NH | 4-stilbenyl | 2 | NMe2 | ↓ | |||||
| 21a | CH2NHSO2 | H | 2 | pyrrol | ||||||
| 21b | CH2NHSO2 | H | 2 | morph | ||||||
| 21c | CH2NHSO2 | H | 2 | NMe2 | ↑ | |||||
| 21d | CH2NHSO2 | 4-Br | 2 | morph | ||||||
| 21e | CH2NHSO2 | 4-Br | 2 | pyrrol | ↑ | ↑ | ↑ | ↑ | ||
| 21f | CH2NHSO2 | 4-Br | 2 | NMe2 | ↑ | |||||
| 21g | CH2NHSO2 | 4-Me | 2 | morph | ||||||
| 21h | CH2NHSO2 | 4-Me | 2 | pyrrol | ↑ | ↑ | ↑ | |||
| 21i | CH2NHSO2 | 4-Me | 2 | NMe2 | ↑ | ↑ | ||||
| 21j | CH2NHSO2 | 4-NO2 | 2 | morph | ||||||
| 21k | CH2NHSO2 | 4-NO2 | 2 | pyrrol | ||||||
| 21l | CH2NHSO2 | 4-NO2 | 2 | NMe2 | ↓ | ↓ | ↓ | ↓ | ↓ | |
| 21m | CH2NHSO2 | 4-F | 2 | morph | ↓ | ↓ | ↓ | ↓ | ||
| 21n | CH2NHSO2 | 4-F | 2 | pyrrol | ↓ | ↓ | ↓ | |||
| 21o | CH2NHSO2 | 4-F | 2 | NMe2 | ↓ | |||||
| 21p | CH2NHSO2 | 4-Me | 2 | diam | ↓ | ↓ | ↓ | ↓ | ||
| 21q | CH2NHSO2 | 4-Br | 2 | diam | ↓ | ↓ | ↓ | |||
| 21r | CH2SO2NH | ‘coumarin’ | 2 | morph | ↓ | ↓ | ||||
| 21s | CH2SO2NH | ‘coumarin’ | 2 | pyrrol | ↓ | ↓ | ||||
| 21t | CH2SO2NH | ‘coumarin’ | 2 | NMe2 | ↓ | ↓ | ||||
| 23a | NHSO2 | H | 2 | NMe2 | ↓ | |||||
| 23b | NHSO2 | H | 2 | pyrrol | ↓ | ↓ | ||||
| 23c | NHSO2 | H | 2 | morph | ||||||
| 23d | NHSO2 | 4-F | 2 | NMe2 | ↓ | ↑ | ||||
| 23e | NHSO2 | 4-F | 2 | pyrrol | ↓ | |||||
| 23f | NHSO2 | 4-F | 2 | morph | ↓ | ↑ | ||||
| 23g | NHSO2 | 4-F | 2 | diam | ↓ | ↑ | ||||
| 23h | NHSO2 | H | 2 | diam | ↓ | ↑ | ||||
| 24a | CH2CONH | H | 2 | NMe2 | ↓ | ↓ | ||||
| 24b | CH2CONH | H | 2 | pyrrol | ↓ | ↓ | ↓ | ↓ | ↓ | |
| 24c | CH2CONH | H | 2 | morph | ↓ | ↓ | ↓ | |||
| 24d | CH2CONH | H | 3 | NMe2 | ||||||
| 24e | CH2NHCO | 5-isoquinolyl | 2 | morph | ↓ | |||||
| 25a | CONH | 3-MeO-C6H4 | 2 | NMe2 | ↓ | ↓ | ↓ | |||
| 25b | CONH | 3-MeO-C6H4 | 2 | pyrrol | ↓ | ↓ | ↓ | |||
| 25c | CONH | 3-MeO-C6H4 | 2 | Morph | ↓ | ↓ | ↓ | |||
| 25d | CONH | 3-MeO-C6H4 | 3 | NMe2 | ↓ | ↑ | ↓ | |||
Bone marrow-derived macrophages preincubated for 18 h with 5 μg/mL of compounds were stimulated with 100 ng/mL LPS, 10 ng/mL BLP, or 0.01 μM CpG in the continued presence of the compounds. After 24 h, supernatants were collected and measured for their IL-12p40 and IL-6 content by ELISA. Arrows down (↓) indicate statistically significant (at least p < 0.05) down-regulation and arrows up (↑) statistically significant up-regulation of the levels of cytokine versus control; blank squares = no significant change. Abbreviations used in structural formulas: ‘coumarin’ = (7-methoxy-2-oxo-2H-chromen-4-yl)methyl; diam = N,N,N-1,1,2-tetramethylethylenediamino2-Me-but = 2-methylbutylamino; 4-Me-pip = 4-methylpiperazinyl; morph = morpholino; 3-OH-n-propyl = 3-hydroxypropylamino; pyrrol = pyrrolidino; stilbenyl = 1-(4-[(E)-2-phenylethyl]benzyl; thiomorph = thiomorpholino.
