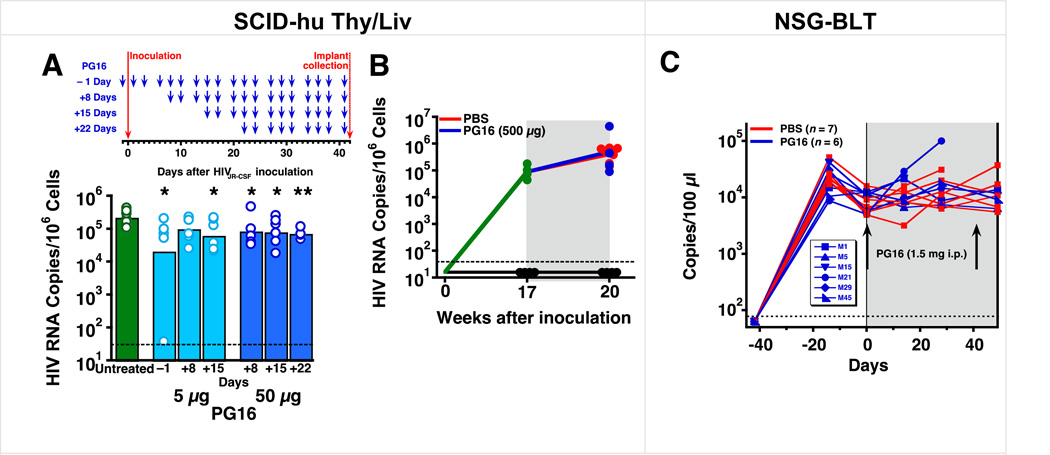
Fig. 6. PG16 had substantially reduced activity in SCID-hu Thy/Liv mice when treatment was initiated 8 days or more after HIVJR-CSF inoculation and had no significant activity in both SCID-hu Thy/Liv mice and NSG-BLT mice with established HIVJR-CSF infection.
(A) HIV RNA was reduced by 1 log10 in SCID-hu Thy/Liv mice treated i.p. with 5 µg PG16 three times per week beginning the day before inoculation and continuing until implant collection at 42 days. Smaller reductions in HIV RNA were observed when treatment was delayed until 8 or more days after inoculation. The columns represent means, and the open circles represent individual mice. **P<0.01 and *P<0.05 compared to untreated HIV-infected mice by the Mann-Whitney U test. (B) No reduction in HIV RNA in SCID-hu Thy/Liv mice treated i.p. with high-dose (500 µg) PG16 or PBS three times per week for 3 weeks beginning 17 weeks after HIVJR-CSF inoculation. The dotted line indicates the HIV RNA detection limit. (101.5 copies per 106 implant cells). (C) Viremic NSG-BLT mice were treated with 1.5 mg PG16 at 6 and 12 weeks after intravaginal HIVJR-CSF inoculation. Each line represents an individual mouse, and sequence analysis of viral RNA from the spleens of PG16-treated mouse #5 and #29 (Supplementary Table 13) revealed Env mutation T162N (data not shown). Mouse #15 died and mouse #21 was euthanized with clinical signs consistent with graft-versus-host disease.