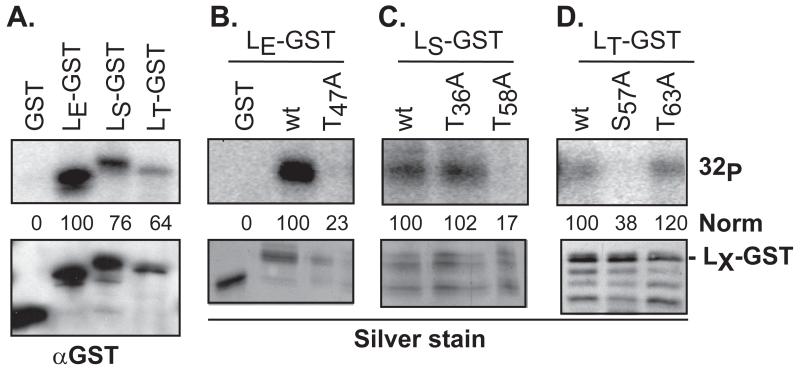
Figure 3.
Phosphorylation of LX-GST by rAMPK. Recombinant GST and LX-GST proteins were reacted with rAMPK (50 ng) and [γ-32P]-ATP as in Methods. After pulldown and protein fractionation, gel bands were detected by phosphoimaging (32P) and silver stain, or after transfer to membranes, by Western analyses (αGST). The phosphorylation signals as measured by densitometry were normalized (norm) to the top band (full-length fusion protein) of LE-GST (A, B) LS-GST (C), or LT-GST (D) αGST signal. Other GST-reactive bands in these panels are from inappropriate LX translational start sites during bacterial protein expression.