Abstract
Objectives
The effects of bone morphogenetic protein-2 (BMP-2) and enamel matrix derivative (EMD) respectively with mineral trioxide aggregate (MTA) on hard tissue regeneration have been investigated in previous studies. This study aimed to compare the osteogenic effects of MTA/BMP-2 and MTA/EMD treatment in MC3T3-E1 cells.
Materials and Methods
MC3T3-E1 cells were treated with MTA (ProRoot, Dentsply), BMP-2 (R&D Systems), EMD (Emdogain, Straumann) separately and MTA/BMP-2 or MTA/EMD combination. Mineralization was evaluated by staining the calcium deposits with alkaline phosphatase (ALP, Sigma-Aldrich) and Alizarin red (Sigma-Aldrich). The effects on the osteoblast differentiation were evaluated by the expressions of osteogenic markers, including ALP, bone sialoprotein (BSP), osteocalcin (OCN), osteopontin (OPN) and osteonectin (OSN), as determined by reverse-transcription polymerase chain reaction analysis (RT-PCR, AccuPower PCR, Bioneer).
Results
Mineralization increased in the BMP-2 and MTA/BMP-2 groups and increased to a lesser extent in the MTA/EMD group but appeared to decrease in the MTA-only group based on Alizarin red staining. ALP expression largely decreased in the EMD and MTA/EMD groups based on ALP staining. In the MTA/BMP-2 group, mRNA expression of OPN on day 3 and BSP and OCN on day 7 significantly increased. In the MTA/EMD group, OSN and OCN gene expression significantly increased on day 7, whereas ALP expression decreased on days 3 and 7 (p < 0.05).
Conclusions
These results suggest the MTA/BMP-2 combination promoted more rapid differentiation in MC3T3-E1 cells than did MTA/EMD during the early mineralization period.
Keywords: Bone morphogenetic protein-2 (BMP-2), Enamel matrix derivative (EMD), MC3T3-E1 cell, Mineral trioxide aggregate (MTA)
Introduction
The regeneration of injured periodontium, including the periodontal ligament, cementum and surrounding bone is essential for optimal healing after perforation repair or apical surgery.1 The crucial role of endodontic cement is to seal the root canal system hermetically and to induce the regenerative potential of adjacent tissues. A number of materials have been introduced as perforation repairing or root-end filling materials, but each of these materials has certain limitations. Mineral trioxide aggregate (MTA) has been reported to fulfill many of the ideal properties of an endodontic filling material.2,3 Nevertheless, MTA also has several shortcomings, including poor handling properties and a long setting time, which can cause an initial washout phenomenon.4 Occasionally, MTA also causes inflammatory and necrotic changes in neighboring tissues during hard tissue formation, which implies the need to further develop less toxic and more bioactive agents.5
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor beta (TGF-β) family, which plays pivotal roles in the commitment and differentiation of cells in the osteoblastic lineage.6 Among the BMP members, BMP-2 is a potent stimulator of osteogenesis in vitro and in vivo.7,8 Moreover, BMP-2 promotes osteoblast maturation by increasing the expression of certain transcription factors and osteoblast marker genes.9 In our previous study, we showed that BMP-2 reduces the initial cytotoxicity of freshly mixed MTA in vitro.10 Several BMPs have been tested experimentally, with the aim of inducing reparative dentinogenesis in dental pulp.11
During tooth development, cells from Hertwig's epithelial root sheath secrete enamel matrix derivative (EMD) onto the newly formed root dentin surface.12 Cementogenesis is a critical event in the repair of endodontic perforations, and EMD has been reported to induce cementogenesis when used for periodontal disease.13 The findings in our other study confirmed the capacity of MTA mixed with EMD to accelerate cementoblastic activity more than MTA alone and that EMD significantly stimulates the mineralization of human cementum-derived cells.14 The results of several previous studies have also suggested the beneficial effects of BMP-2 and EMD on hard tissue repair and regeneration. However, to the best of our knowledge, there has been no study comparing the combined effect of MTA/BMP-2 and MTA/EMD. The purpose of this study was to compare the effects of MTA/BMP-2 and MTA/EMD on promoting hard tissue repair and regeneration in MC3T3-E1 cells.
Materials and Methods
Cell culture
Murine preosteoblastic clone cells, MC3T3-E1, were used in this study. These cells were obtained from American Type Culture Collection (ATCC, CRL-1427, Manassas, VA, USA). Immediately upon arrival, cells were thawed and suspended in a 100 mm culture dish with alpha minimum essential medium (α-MEM, Gibco, Grand Island, NY, USA). The cells were thawed and suspended in a 100-mm culture dish and cultured in α-MEM supplemented with 10% (v/v) fetal bovine serum (Gibco), 1% (w/v) antibiotics/antimycotics (the stock solution contained 100 U penicillin G sodium/mL, 100 µg/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B, in saline), 1.0 mmol/L sodium pyruvate, 0.1 mmol/L nonessential amino acids, and 1.5 g/L sodium bicarbonate (Gibco) at 37℃ in an a humidified atmosphere containing 5% (v/v) CO2. After establishing cultures from frozen cells, they were sub-cultured several times. Confluent cells were detached using 0.25% trypsin in Mg2+ and Ca2+ free phosphate-buffered saline before use.
Preparation of test materials and cell treatments
MTA (ProRoot, Dentsply, Tulsa, OK, USA) was mixed aseptically in accordance with the manufacturer's instructions and then packed into a Teflon mold of 9.5 mm in diameter and 2 mm in thickness. They were allowed to set for 24 hours in a humidified incubator at 37℃ and then placed on the bottom of the culture plate inserts (Millicell, Millipore, Bedford, MA, USA) of six-well culture plates, which had a membrane pore diameter of 0.4 mm. They were then rinsed three times with 70% ethanol and immersed in serum-free Dulbecco's modified Eagle's Medium (DMEM, Invitrogen, Carlsbad, CA, USA, 2 mL/well) for an additional 72 hours. EMD gel (Emdogain, Straumann, Basel, Switzerland), a commercial preparation of porcine fetal EMD, was diluted in sterile distilled water to a concentration of 100 µg/mL.13 One µg of recombinant human BMP-2 (R&D Systems, Minneapolis, MN, USA) was used in each well for the BMP-2 group.
The cells were seeded onto six-well culture plates at an initial density of 5,000 cells/cm2. After 24 hours of incubation, the cells from each group were subjected to different treatments as described below and incubated for 3 or 7 more days. EMD and BMP-2 were placed on the set MTA specimen for groups 5 and 6, respectively.
Group 1. media and insert only (negative control)
Group 2. EMD
Group 3. BMP-2
Group 4. MTA
Group 5. MTA and EMD
Group 6. MTA and BMP-2
Alkaline phosphatase (ALP) staining
MC3T3-E1 cells were seeded onto 24-well culture plate at a density of 50,000 cells/well and incubated for 24 hours. And set MTA specimen on the inserts in MTA-containing groups and other agents such as BMP-2 and EMD with or without MTA also were treated to the cells and were cultured for additional 7 days. The mineralizing medium was replaced every other day. ALP staining kit (86R-1KT, Sigma-Aldrich, St. Louis, MO, USA) was used under the instructions of manufacturer. Sodium nitrate solution 1 mL and FRV-alkaline solution 1 mL were mixed and incubated for 2 minutes at room temperature. The mixed solution was diluted with 45 mL of double distilled water and supplemented with 1 mL of naphthol AS-BI alkaline solution resulting in alkaline-dye mixture. The cultured cells were washed with cold phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde for 30 minutes at room temperature. After the fixation procedure, the cells were washed three times with PBS and washed again with distilled water. Alkaline-dye mixture was added by 200 µL/well and the plate was wrapped with foil and incubated for 20 minutes at 37℃ under dark condition.
Alizarin red staining
After 24 hours of the initial cell culture to enable cell attachment, the growth medium was substituted for mineralizing medium, which was α-MEM containing 2% fetal bovine serum, supplemented with ascorbic acid (50 mg/mL) and β-glycerophosphate (10 mmol/L). This mineralizing medium was replaced on day 3, and calcium accumulation on day 7 was assessed using Alizarin red (Sigma-Aldrich) staining. Forty mmol/L of Alizarin red, buffered with ammonium hydroxide to pH 4.2, was prepared in distilled water and applied to the cells in the six-well plates for 30 minutes at room temperature with gentle agitation. The cells were then rinsed with distilled water and allowed to dry. Calcified nodules with bright red color were photographed.
Reverse-transcriptase polymerase chain reaction (RT-PCR)
The expression of mineralization markers ALP, bone sialoprotein (BSP), osteocalcin (OCN), osteopontin (OPN) and osteonectin (OSN) was assessed using optimal primers designed with Primer express software. The primer sequences for each marker and for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control are detailed in Table 1.
Table 1.
Primer sequences for each mineralizing marker and GAPDH for RT-PCR analysis
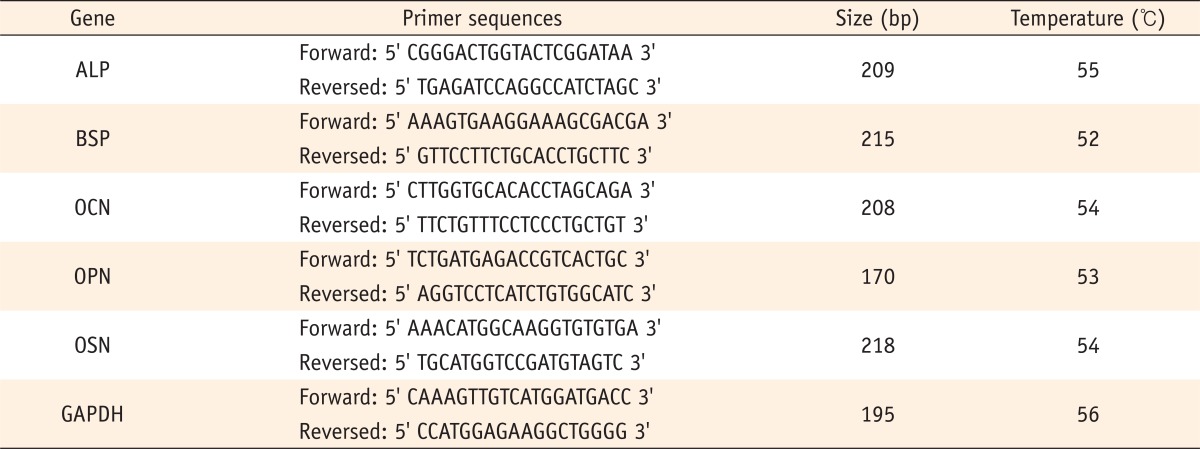
ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN, osteocalcin; OPN, osteopontin; OSN, osteonectin, GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse-transcription polymerase chain reaction analysis.
After 3 or 7 days of culture, the total RNA was extracted from the MC3T3-E1 cells using Trizol reagent (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer's instructions. RNA reverse transcription was performed using AccuPower RT PreMix (Bioneer, Daejeon, Korea). The RT-generated DNA (2 - 5 µL) was amplified using an AccuPower PCR PreMix (Bioneer) for 30 cycles in a DNA thermal cycler. The PCR products were resolved on a 1.5% agarose gel and stained with ethidium bromide. The intensity of each band was normalized with GAPDH and quantified on the photographed gels using a densitometer (Quantity One, Bio-Rad, Hercules, CA, USA).
Statistical analysis
A statistical analysis of the data was performed using a one-way analysis of variance followed by a Duncan test using the SPSS program (SPSS 12.0, SPSS GmbH, Munich, Germany). Statistical significance was set at p < 0.05.
Results
Alkaline phosphatase staining
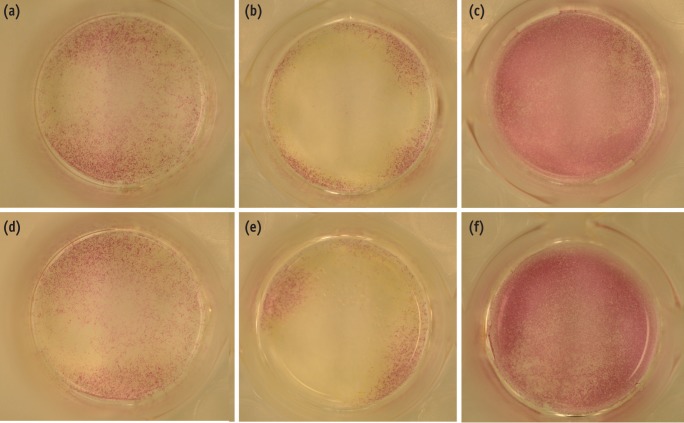
ALP staining for detecting ALP activity in cells revealed that the expression of ALP decreased largely in EMD-treated group with or without MTA than any other experimental or control group (Figure 1).
Figure 1.
Alkaline phosphatase (ALP) expression in MC3T3-E1 cells in control and experimental groups. MC3T3-E1cells were cultured with or without treated materials for 7 days and stained with alkaline-dye mixture provided by an ALP staining kit. A representative photograph of ALP staining is shown, (a) control; (b) EMD; (c) BMP-2; (d) MTA; (e) MTA/EMD; (f) MTA/BMP-2. EMD, enamel matrix derivative; BMP, bone morphogenetic protein; MTA, mineral trioxide aggregate.
Alizarin red staining
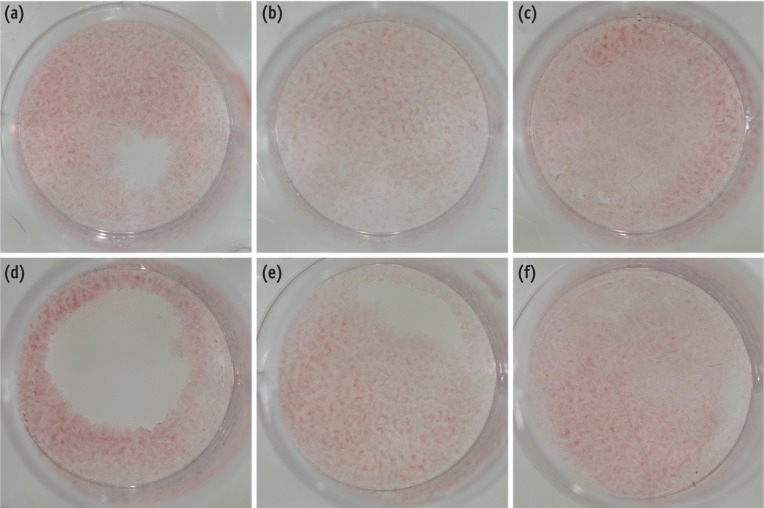
Alizarin red staining for calcium revealed largely increased mineralization in the BMP-2- and MTA/BMP-2-treated groups and increased to a lesser extent in the EMD and MTA/EMD groups. However, the amount of mineralization showed a trend toward decreasing in the MTA-only cells compared to the negative control cells (Figure 2).
Figure 2.
Formation of calcification nodules in each group in MC3T3-E1 cells. MC3T3-E1 cells were cultured with or without treated materials for 7 days and stained with Alizarin red. A representative photograph of Alizarin red staining is shown, (a) control; (b) EMD; (c) BMP-2; (d) MTA; (e) MTA/EMD; (f) MTA/BMP-2. EMD, enamel matrix derivative; BMP, bone morphogenetic protein; MTA, mineral trioxide aggregate.
RT-PCR gene expression analysis
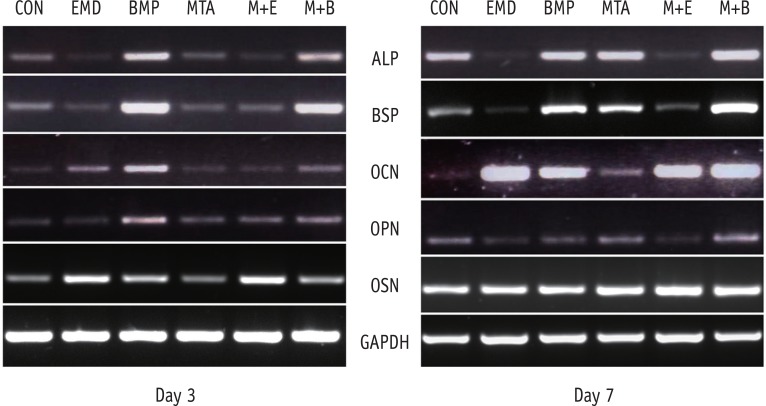
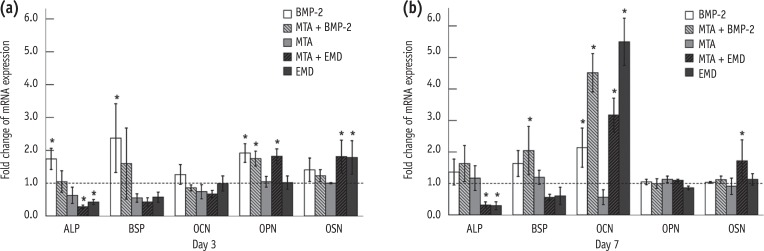
ALP, BSP, OCN, OPN and OSN served as markers of osteoblast differentiation and mineralization. ALP, BSP, OCN, OPN, and OSN gene expression were generally found to be up-regulated in the BMP-2-treated cells on days 3 and 7, but not all of these differences were statistically significant (Figures 3 and 4). Nevertheless, in the MTA/BMP-2 group, the mRNA expression of OPN on day 3 and BSP and OCN on day 7 was significantly increased.
Figure 3.
The effects of BMP-2 and EMD on messenger RNA expression for ALP, BSP, OCN, OPN and OSN in MC3T3-E1 cells on days 3 (a) and 7 (b). The total mRNA was extracted from the cells, and mRNA expression was determined using RT-PCR. CON, control; BMP-2, bone morphogenetic protein-2; EMD, enamel matrix derivative; MTA, mineral trioxide aggregate; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN, osteocalcin; OPN, osteopontin; OSN, osteonectin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse-transcription polymerase chain reaction analysis.
Figure 4.
The fold change in the mRNA expression for ALP, BSP, OCN, OPN and OSN in MC3T3-E1 cells relative to the control on days 3 (a) and 7 (b). The control was assigned an optical density reference value of 1.0 (*p < 0.05). BMP-2, bone morphogenetic protein-2; EMD, enamel matrix derivative; MTA, mineral trioxide aggregate; ALP, alkaline phosphatase; BSP, bone sialoprotein; OCN, osteocalcin; OPN, osteopontin; OSN, osteonectin.
In the MTA/EMD group, OPN and OSN expression were significantly increased on day 3 and OSN and OCN levels were significantly increased on day 7, whereas ALP expression in the EMD and MTA/EMD groups was significantly down-regulated on days 3 and 7. Additionally, OCN expression on day 7 in the EMD-treated group was significantly up-regulated (p < 0.05).
Discussion
Factors, such as BMPs and EMD, play an important role in bioengineering, and there have been a number of reports of their use for tissue regeneration. BMPs are osteogenic factors that were originally identified as the active components within osteo-inductive extracts derived from bone and are capable of stimulating osteoblast differentiation and bone formation at ectopic sites.15,16 A previous report suggested that EMD may also stimulate odontoblasts or pulp cells directly to produce collagen matrix for calcification.17 In this study, we compared the effects of MTA/BMP-2 and MTA/EMD on the hard tissue regeneration potential in vitro. Uniquely, ALP expression in the EMD and MTA/EMD groups was significantly down-regulated on days 3 and 7. This result is consistent with the previous finding of Palioto et al. that EMD had an inhibitory effect on ALP activity in human osteoblastic cells but not PDL cells.18 ALP is a potential Ca2+ carrier and is well known to play an important role in osteogenic development by hydrolyzing inhibitors of mineral deposition. ALP activity has been considered to be an early marker of human dental pulp cell mineralization because ALP is generally up-regulated during the initial mineralization stage.19 Wada et al. reported that the growth factors present in EMD play certain roles in regulating its bioactivity and TGF-β suppresses OCN gene expression in osteoblasts.20 The TGF-β family blocks ALP and OCN gene expression during osteoblastic differentiation.21 In addition, the effects of TGF-β on the expression of osteoblastic phenotypes have been shown to be suppressed when osteoblastic cells differentiate into mature stage counterparts, and TGF-β suppresses ALP activity in MC3T3-E1 osteoblastic cells.22 Hence, ALP down-regulation in EMD-treated MC3T3-E1 cells appears to result from the suppressive effects of TGF-β.
OCN, the most abundant non-collagenous bone matrix protein, is a small γ-carboxyglutamate protein preferentially expressed by osteoblasts. OCN may regulate the activities of osteoclasts and their precursor cells and mark the turning point between bone formation and resorption. The exact role of OCN in bone is not yet completely understood, but its ability to regulate bone mineralization and turnover has been reported.23 OCN is a major protein synthesized by osteoblasts, odontoblasts, and cementoblasts.11 In our present study, OCN was found to be strongly up-regulated in BMP-2- or EMD-treated cells after seven days. Our findings are consistent with a previous report by Reseland et al. which showed that OCN expression was up-regulated in EMD-treated osteoblastic cells.24 Among these genes, BSP is produced primarily by osteoblasts, is considered to be a marker of the osteoblastic phenotype and may serve as a matrix-associated signal that directly promotes osteoblast differentiation, resulting in increased production of a mineralized matrix.25,26 And the expressions of BSP and ALP on day 3 in MTA/BMP-2 group were significantly down-regulated than in BMP-2-only group. These findings can be explained by the expected inhibitory effect of MTA on the osteogenic activity of BMP-2. But this tendency was found to change on day 7. Therefore to clarify the interaction of MTA and BMP-2 affecting the expressions of such osteogenic gene markers, we think additional studies need to be performed. OPN is a secreted glycophosphoprotein and is found in both mineralized and non-mineralized tissues. Based on its strong inhibition of hydroxyapatite formation in vitro, OPN is believed to play a crucial role in modulating the apatite crystal growth of bone.27 OPN has been reported to be required for stress-induced bone remodeling, as in ovariectomy or ectopic bone disc implantation.28 The up-regulation of OPN expression by MTA/EMD has been demonstrated to be time dependent in murine cementoblasts and MG-63 cells, respectively.29,30 In our current study, OPN expression was found to be up-regulated in the MTA/BMP-2 and MTA/EMD groups on day 3, suggesting that the mineralization induced by combination of MTA and BMP-2 or EMD in MC3T3-E1 cells may promote OPN expression during an early stage. These findings are consistent with the results of a previous study that OPN is up-regulated in EMD-treated MTA groups on day 3.14 In our previous study, we found that MTA and EMD significantly stimulated the mineralization of human cementum-derived cells, which is consistent with the findings of Weishaupt et al. who reported that the beneficial periodontal regeneration after treatment with EMD may be related to increased expression of the mineralization markers BSP and OPN at the mRNA level.31
OSN is a major non-collagenous matrix protein in bone and dentin and is found in bovine odontoblasts and predentin from human prenatal and postnatal samples.32 OSN is associated spatially and temporally with development, tissue remodeling, and repair and may mediate deposition of hydroxyapatite, bind to growth factors, and influence the cell cycle. OSN is also a positive regulator of bone formation. In our present study, we found that OSN expression was up-regulated in the EMD and MTA/EMD groups on day 3. This finding is consistent with data from Papagerakis et al. who previously reported that OSN is expressed in the initial stages of the cyto-differentiation of tooth development, whereas OCN was only expressed during the later stages.33 Differences in the timing of mRNA up-regulation may indicate different roles in MTA/EMD-induced mineralization.34 However, further studies with longer observation periods are needed to properly assess this hypothesis.
Conclusions
Within the limitations of our present study, we conclude that the MTA/BMP-2 combination promoted more rapid differentiation in MC3T3-E1 cells than MTA/EMD-treated cells during the early mineralization period. Further studies are needed to elucidate the exact mechanisms underlying the influence of these agents on hard tissue regeneration and their direct interaction with surrounding tissues over a longer period of time.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35:321–328. doi: 10.1016/j.joen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RS, Mauger M, Clement DJ, Walker WA., 3rd Mineral trioxide aggregate: a new material for endodontics. J Am Dent Assoc. 1999;130:967–975. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225–228. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 4.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Min KS, Yang SH, Kim EC. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endod. 2009;35:847–851. doi: 10.1016/j.joen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ, Suda T, Yoshiki S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991;113:681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, Wozney JM, Li XJ. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13:1483–1490. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 9.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 10.Ko H, Yang W, Park K, Kim M. Cytotoxicity of mineral trioxide aggregate (MTA) and bone morphogenetic protein 2 (BMP-2) and response of rat pulp to MTA and BMP-2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e103–e108. doi: 10.1016/j.tripleo.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima M. Induction of dentin formation on canine amputated pulp by recombinant human bone morphogenetic proteins (BMP)-2 and -4. J Dent Res. 1994;73:1515–1522. doi: 10.1177/00220345940730090601. [DOI] [PubMed] [Google Scholar]
- 12.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Safavi KE, Spangberg LS, Zhu Q. Enamel matrix derivative prolongs primary osteoblast growth. J Endod. 2001;27:110–112. doi: 10.1097/00004770-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Chung H, Yang W, Kim M, Ko H. Comparison of the effects of enamel matrix derivative and mineral trioxide aggregate on the mineralization potential of human cementum-derived cells. J Dent Sci. 2011;6:153–157. [Google Scholar]
- 15.Wozney JM. The bone morphogenetic protein family: multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci. 1998;106(Supplement 1):160–166. doi: 10.1111/j.1600-0722.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 16.Lianjia Y, Yan J, Doi T, Sekine I, Ogawa K, Mori M. Immunohistochemical localization of bone morphogenetic protein (BMP) in calcifying fibrous epulis. J Oral Pathol Med. 1993;22:406–410. doi: 10.1111/j.1600-0714.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Min KS, Choi GW, Park JH, Park SH, Lee SI, Kim EC. Effects of simvastain and enamel matrix derivative on Portland cement with bismuth oxide-induced growth and odontoblastic differentiation in human dental pulp cells. J Endod. 2012;38:405–410. doi: 10.1016/j.joen.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Palioto DB, Rodrigues TL, Marchesan JT, Beloti MM, de Oliveira PT, Rosa AL. Effects of enamel matrix derivative and transforming growth factor-β1 on human osteoblastic cells. Head Face Med. 2011;7:13. doi: 10.1186/1746-160X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada Y, Yamamoto H, Nanbu S, Mizuno M, Tamura M. The suppressive effect of enamel matrix derivative on osteocalcin gene expression of osteoblasts is neutralized by an antibody against TGF-β. J Periodontol. 2008;79:341–347. doi: 10.1902/jop.2008.070197. [DOI] [PubMed] [Google Scholar]
- 21.Ibbotson KJ, Orcutt CM, Anglin AM, D'Souza SM. Effects of transforming growth factors beta 2 on a mouse clonal, osteoblastlike cell line MC3T3-E1. J Bone Miner Res. 1989;4:37–45. doi: 10.1002/jbmr.5650040107. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno M, Fujisawa R, Kuboki Y. Carboxyl-terminal propeptide of type I collagen (c-propeptide) modulates the action of TGF-β on MC3T3-E1 osteoblastic cells. FEBS Lett. 2000;479:123–126. doi: 10.1016/s0014-5793(00)01900-1. [DOI] [PubMed] [Google Scholar]
- 23.Neve A, Corrado A, Cantatore FP. Ostecalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228:1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- 24.Reseland JE, Reppe S, Larsen AM, Berner HS, Reinholt FP, Gautvik KM, Slaby I, Lyngstadaas SP. The effect of enamel matrix derivative on gene expression in osteoblasts. Eur J Oral Sci. 2006;114(Supplement 1):205–211. doi: 10.1111/j.1600-0722.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno M, Imai T, Fujisawa R, Tani H, Kuboki Y. Bone sialoprotein (BSP) is a crucial factor for the expression of osteoblastic phenotypes of bone marrow cells cultured on type I collagen matrix. Calcif Tissue Int. 2000;66:388–396. doi: 10.1007/s002230010078. [DOI] [PubMed] [Google Scholar]
- 26.Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 27.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 28.Ishijima M, Tsuji K, Rittling SR, Yamashita T, Kurosawa H, Denhardt DT, Nifuji A, Ezura Y, Noda M. Osteopontin is required for mechanical stress-dependent signals to bone marrow cells. J Endocrinol. 2007;193:235–243. doi: 10.1677/joe.1.06704. [DOI] [PubMed] [Google Scholar]
- 29.Swanson EC, Fong HK, Foster BL, Paine ML, Gibson CW, Snead ML, Somerman MJ. Amelogenins regulate expression of genes associated with cementoblasts in vitro. Eur J Oral Sci. 2006;114(Supplement 1):239–243. doi: 10.1111/j.1600-0722.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen CL, Huang TH, Ding SJ, Shie MY, Kao CT. Comparison of calcium and silicate cement and mineral trioxide aggregate biologic effects and bone markers expression in MG63 cells. J Endod. 2009;35:682–685. doi: 10.1016/j.joen.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Weishaupt P, Bernimoulin JP, Trackman P, Hägewald S. Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:304–308. doi: 10.1016/j.tripleo.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 32.Fujisawa R, Kuboki Y. Changes in levels of osteonectin in bovine dentine during tooth development. Arch Oral Biol. 1989;34:89–92. doi: 10.1016/0003-9969(89)90131-3. [DOI] [PubMed] [Google Scholar]
- 33.Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J, Simmer J, Macdougall M. Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone. 2002;30:377–385. doi: 10.1016/s8756-3282(01)00683-4. [DOI] [PubMed] [Google Scholar]
- 34.Kim SG, Ryu SI. Enamel matrix derivative for replanted teeth in animal models: a systematic review and meta-analysis. Restor Dent Endod. 2013;38:194–203. doi: 10.5395/rde.2013.38.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]