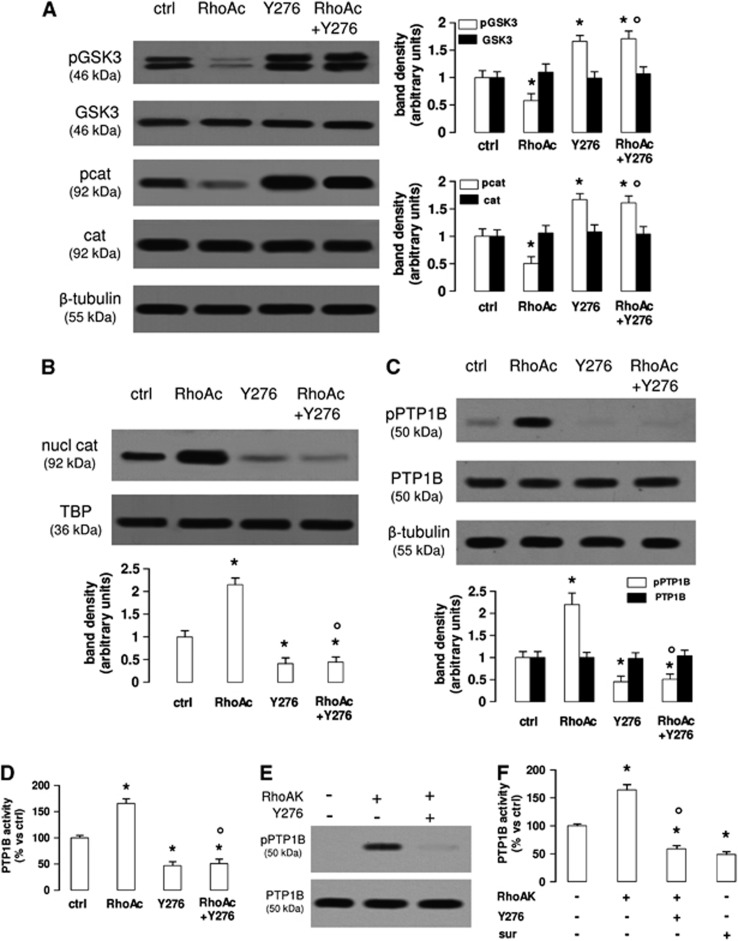
Figure 3.
The RhoA kinase (RhoAK) inhibition increases the activation of glycogen synthase kinase 3 (GSK3), by decreasing the activity of protein tyrosine phosphatase 1B (PTP1B) in human blood–brain barrier cells. The hCMEC/D3 cells were grown in fresh medium (ctrl) or in medium containing the RhoA activator II (RhoAc; 5 μg/mL for 3 hours) or the RhoAK inhibitor Y27632 (Y276; 10 μmol/L for 3 hours), alone or co-incubated. (A) Western blot analysis of phospho(Tyr216)GSK3 (pGSK3), GSK3, phospho(Ser33/Ser37/Thr41)β-catenin (pcat), β-catenin (cat) in whole-cell lysates. The β-tubulin expression was used as a control of equal protein loading. The figure is representative of three experiments with similar results. The band density ratio between each protein and β-tubulin was expressed as arbitrary units. Versus ctrl cells: *P<0.05; versus RhoAc alone: °P<0.001. (B) The nuclear extracts were analyzed for the amount of β-catenin (nucl cat). The expression of TATA-binding protein (TBP) was used as a control of equal protein loading. The band density ratio between each protein and TBP was expressed as arbitrary units. Versus ctrl cells: *P<0.002; versus RhoAc alone: °P<0.001. (C) Western blot analysis of phospho(Ser50)PTP1B (pPTP1B) and PTP1B. The β-tubulin expression was used as a control of equal protein loading. The figure is representative of three experiments with similar results. The band density ratio between each protein and β-tubulin was expressed as arbitrary units. Versus ctrl cells: *P<0.005; versus RhoAc alone: °P<0.001. (D) The activity of endogenous PTP1B was measured in cell lysates, as reported under Materials and Methods. Data are presented as means±s.d. (n=3). Versus ctrl: *P<0.002; versus RhoAc alone: °P<0.001. (E) In vitro phosphorylation of PTP1B in the presence of RhoAK and Y27632. 5 U of human recombinant PTP1B were incubated in the absence (−) or in the presence of 10 U of human recombinant RhoAK, alone or in the presence of the RhoAK inhibitor Y27632 (Y276; 10 μmol/L) for 30 minutes at 37°C, in a reaction buffer containing 25 mmol/L ATP. At the end of this incubation time, samples were resolved by SDS–PAGE and probed with anti-phospho(Ser50)PTP1B (pPTP1B) or anti-PTP1B antibodies. The figure is representative of three experiments with similar results. (F) The activity of purified PTP1B was measured in a cell-free system, using a recombinant phospho(Tyr 216)GSK3 peptide as substrate. When indicated, 10 U of RhoAK, alone or in the presence of Y27632 (Y276; 10 μmol/L), were added in the reaction mix 30 minutes before adding the phospho(Tyr 216)GSK3 peptide. Suramin (sur; 10 μmol/L), a known inhibitor of PTP1B, was added together with the phospho(Tyr 216)GSK3 peptide, as internal control. Data are presented as means±s.d. (n=3). Versus PTP1B alone (−): *P<0.002; versus RhoAK: °P<0.001.